Advanced Regenerative Medicine Focused Only on 'Industry,' Neglecting 'Public Health'...
"A Second Invossa Scandal Could Happen"
Everyone Racing for 'Advanced Regenerative' Certification...
Even Professional Brokers Appear
Non-Reimbursed and Indemnity Reform Plans Rendered Ineffective...
Government Virtually Abandons Control Over Non-Reimbursed Expansion
Advanced regenerative medicine is rapidly emerging as a hidden risk factor that could further deepen the financial deficit of indemnity health insurance. This is because a flood of non-reimbursed treatments lacking clinical evidence may soon enter the medical field under the guise of "innovation." Experts warn that the non-reimbursed and indemnity insurance reform plan announced earlier this year is likely to become ineffective, and they advise the new administration to urgently devise countermeasures.
Advanced Regenerative Medicine Focused Solely on 'Industry,' Neglecting 'Public Health'... "A Second Invossa Scandal Could Happen"
In recent years, there has been a growing trend of Korean patients with severe, rare, or intractable diseases traveling to Japan to receive stem cell injections. Each injection costs up to 8 million won, and approximately 30,000 people travel abroad annually for these treatments. The medical community has called for reforms, arguing that regulations on stem cell therapy in Korea became excessively strict after the so-called "Hwang Woo-suk scandal" in 2005, causing patients to spend their medical expenses overseas. During the Yoon Suk-yeol administration, related legislation was pushed forward despite conflicts with the medical community in an attempt to address this issue. As a result, the amendment to the "Act on the Safety and Support of Advanced Regenerative Medicine and Advanced Biopharmaceuticals (Advanced Regenerative Medicine Act)" took effect in February, paving the way for the widespread adoption of advanced regenerative therapies in Korea.
Advanced regenerative medicine refers to medical technologies that restore, maintain, or improve the function of damaged tissues or organs. Representative examples include stem cell therapy, immune cell therapy, and gene therapy. The amendment to the Advanced Regenerative Medicine Act expanded the scope of clinical research subjects from only severe, rare, and intractable diseases to all diseases. It also allows patients with severe, rare, or intractable conditions to receive treatment during the clinical research phase without going through phase 3 clinical trials, as long as safety and efficacy are confirmed, and without approval from the Ministry of Food and Drug Safety. This means patients can now receive treatment domestically instead of traveling to Japan, and the costs can be covered by indemnity insurance.
The problem is that, similar to new medical technologies such as stem cell knee injections?which have recently sparked controversy over overtreatment?non-reimbursed advanced regenerative medicine allows hospitals to set prices at their own discretion. Although the government claims it will review treatment plans and costs through the Advanced Regenerative Medicine and Advanced Biopharmaceuticals Review Committee, even six months after the amendment's implementation, there are still no concrete standards for appropriate pricing. Kim Jaeheon, Secretary General of the Free Medical Care Movement Headquarters, said, "There are many figures from the advanced regenerative medicine sector on the review committee, so it is questionable whether proper reviews will be conducted," adding, "There is a risk of a repeat of the Invossa scandal, which caused significant social turmoil due to the lax review by interested parties."
Everyone Racing for 'Advanced Regenerative' Certification... Even Professional Brokers Appear
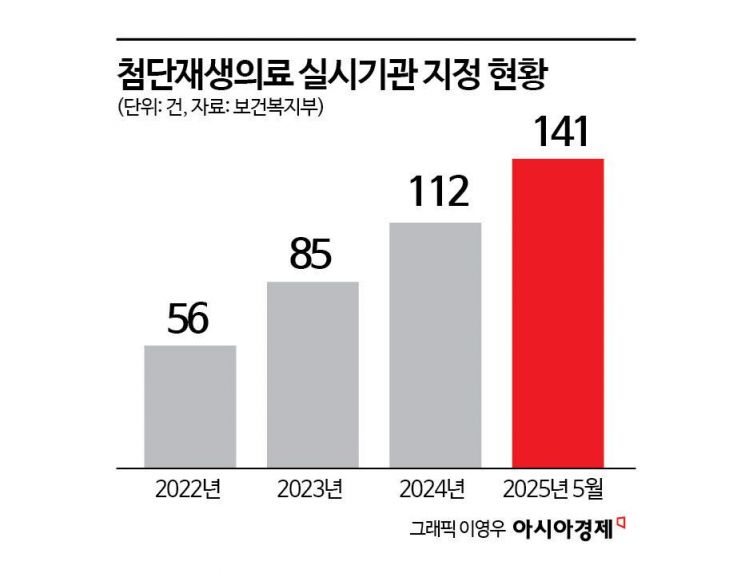
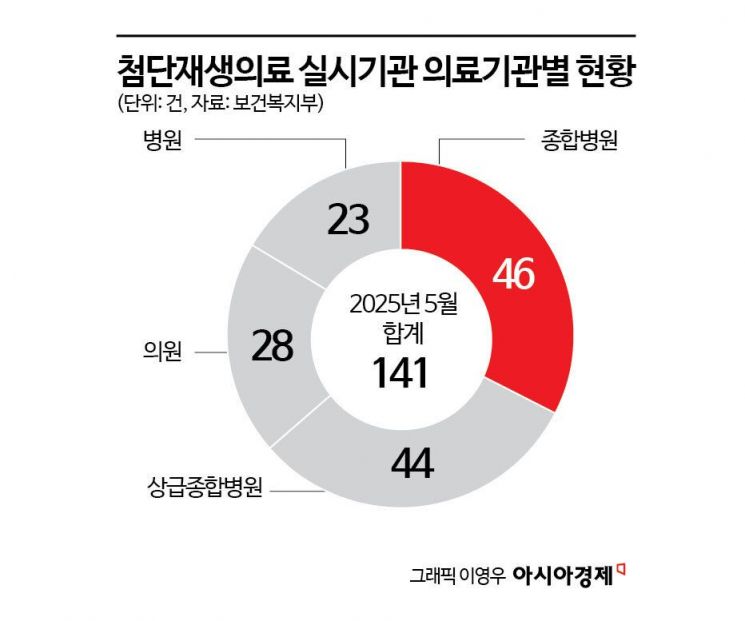
Currently, medical institutions such as clinics, hospitals, and Korean medicine hospitals are rushing to introduce advanced regenerative medicine by exploiting institutional loopholes. In order to perform advanced regenerative medical procedures, institutions must be designated by the Ministry of Health and Welfare as advanced regenerative medicine providers. As of May, there were 141 designated institutions. While about 30 institutions were designated each year from 2022 to 2024, this year alone, over 30 were added in just five months. By type, general hospitals (46), tertiary hospitals (44), clinics (28), and hospitals (23) had the most designations, with a significant number of plastic surgery, dermatology, and Korean medicine hospitals as well. Regionally, the majority were concentrated in the Seoul metropolitan area: Seoul (60), Gyeonggi (29), Busan (13), and Incheon (10).
Professional brokers disguised as consultants have already appeared. Internet blogs and medical communities are flooded with advertisements offering to handle the designation process for advanced regenerative medicine institutions and to create business portfolios. One blog enticed readers by claiming that designation as an advanced regenerative medicine institution would enable "expansion of high-profit non-reimbursed treatment models," "increased credibility and branding as a government-certified institution," and "attracting foreign patients." They also promoted their services for meeting detailed requirements such as facilities, equipment, and personnel, as well as assisting with the document preparation process.
Many experts, including those in the medical community, unanimously express concern that the influx of unverified medical technologies, equipment, and pharmaceuticals into the market could jeopardize patient health above all else. Normally, pharmaceutical companies must spend significant time and resources to test safety and efficacy through phase 3 clinical trials, but critics argue that the amendment to the Advanced Regenerative Medicine Act shifts these costs onto patients. Lee Donggeun, Director of Policy Planning at the Pharmacists for a Healthy Society, pointed out, "Most advanced regenerative medicine involves harvesting, culturing, and manipulating the patient's own cells before reintroducing them into the body, which is much riskier, yet adequate safety reviews are not being conducted." He added, "Most domestically developed cell therapies approved for advanced regenerative treatment lack sufficient verification and should all undergo clinical re-evaluation." Nam Eunkyung, Head of the Social Policy Team at the Citizens' Coalition for Economic Justice, said, "Patients with severe, rare, or intractable diseases are likely desperate for any hope," but warned, "If a flood of medical technologies that have not even undergone proper clinical trials pours into the market, their health could be put at even greater risk."
In the medical field, advanced regenerative medicine institutions are already promoting "cutting-edge technology" and performing advanced regenerative treatments such as bone marrow knee injections, Cartistem, Immuncell, and autologous bone marrow stem cell therapy. Prices vary widely by institution. According to insurance claim data obtained from a major non-life insurance company, bone marrow knee injections cost between 2 million and 15 million won per case, Cartistem between 5.7 million and 32 million won, Immuncell between 80,000 and 11 million won, and autologous bone marrow stem cell therapy between 20,000 and 15 million won. A hospital-level advanced regenerative medicine institution in Gangnam-gu, Seoul, was caught harvesting bone marrow from a bone marrow cancer patient to perform knee injections. A clinic-level institution in the Seoul metropolitan area induced hospitalizations and overcharged for medical expenses by exaggerating indications and defect areas when administering Cartistem.
There are also concerns that the absence of a legal definition for intractable diseases will create various "loopholes" in indemnity insurance. Currently, severe diseases are managed under the National Health Insurance Act, and rare diseases under the Rare Disease Management Act. However, there is no single law or clear classification concept for intractable diseases. A long-term claims manager at an insurance company commented, "For severe and rare diseases, it is gratifying for insurers when patients receive verified advanced regenerative treatments and get reimbursed through indemnity insurance," but warned, "If relatively common intractable diseases like rhinitis, athlete's foot, or atopic dermatitis are linked to advanced regenerative medicine and abused for cosmetic procedures, the problem could become serious."
Non-Reimbursed and Indemnity Reform Plans Rendered Ineffective... Government Virtually Abandons Control Over Non-Reimbursed Expansion
The Special Committee on Medical Reform and the Financial Services Commission, both operating under the Yoon Suk-yeol administration, announced non-reimbursed and indemnity insurance reform plans twice?once in August last year and again in April this year. The core of the non-reimbursed reform plan is to convert certain controversial non-reimbursed items, such as manual therapy and nutritional injections, into managed benefits. The indemnity insurance reform plan aims to link the coinsurance rate for non-reimbursed outpatient care to the coinsurance rate for health insurance, and to differentiate coverage for non-reimbursed treatments based on whether the case is severe or non-severe.
Experts believe that the non-reimbursed and indemnity insurance reform plans will not be able to effectively control the surge of non-reimbursed treatments in the medical field, which is being driven by the amendment to the Advanced Regenerative Medicine Act and the expansion of early-access medical technologies. Although a "Non-Reimbursed Management Policy Council" was launched in May to manage non-reimbursed items following the reform plan's announcement, there has been no progress in discussions under the new administration. The fifth-generation indemnity insurance plan was originally scheduled for release around the end of the year, but there may be partial revisions because it conflicts with President Lee Jaemyung's campaign pledge for "optional riders." Kim Kyungseon, a research fellow at the Insurance Research Institute, said, "As it stands, even if the non-reimbursed and indemnity insurance reform plans are implemented, the government will only be able to respond reactively to the abuse of non-reimbursed treatments resulting from the expansion of advanced regenerative medicine and early-access medical technologies," adding, "This is because there are no concrete guidelines from health authorities regarding the application of cutting-edge medical technologies, nor is there any control over medical fees."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.


![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














