"While continuing discussions with overseas pharmaceutical companies, what we had been waiting for was the top-line results of the Phase 2a clinical trial. We believe the results exceeded expectations, and we anticipate that discussions will accelerate." (Kim Hoon-taek, CEO of Tium Bio)
 Kim Hoon-taek, CEO of Tium Bio, is presenting the results of the Phase 2a clinical trial of 'Marigolicx' at a briefing held on the afternoon of the 7th in Yeouido, Yeongdeungpo-gu, Seoul.
Kim Hoon-taek, CEO of Tium Bio, is presenting the results of the Phase 2a clinical trial of 'Marigolicx' at a briefing held on the afternoon of the 7th in Yeouido, Yeongdeungpo-gu, Seoul. [Photo by Lee Chun-hee]
The Phase 2a clinical trial results of Marigolicx, Tium Bio's leading pipeline drug for the treatment of endometriosis, have been disclosed in Europe. At a briefing held on the afternoon of the 7th in Yeouido, Yeongdeungpo-gu, Seoul, Kim Hoon-taek, CEO of Tium Bio, explained, "As a gonadotropin-releasing hormone (GnRH) antagonist, significant effects were observed in all treatment groups," adding, "Since we confirmed up to 92% pain suppression effect, Marigolicx is expected to yield favorable clinical results."
Endometriosis, the target indication for Marigolicx, is a condition where endometrial tissue implants and grows outside the uterus, causing pain such as dysmenorrhea and dyspareunia, as well as infertility. It frequently occurs in 10-30% of women of reproductive age and is estimated to affect approximately 200 million patients worldwide. The market size for endometriosis treatments in the Group of Seven (G7) countries is projected to reach $2.7 billion (about 3.7 trillion KRW) by 2030.
Marigolicx works by acting on GnRH receptors to suppress estradiol, a female hormone that worsens endometriosis, thereby providing therapeutic effects. Although new GnRH-based drugs have been developed to overcome the issues of existing male hormone-based drugs that induce temporary menopause, these also have problems such as osteoporosis induction and low response rates. Tium Bio explains that Marigolicx can increase response rates while reducing bone loss compared to existing GnRH-based drugs.
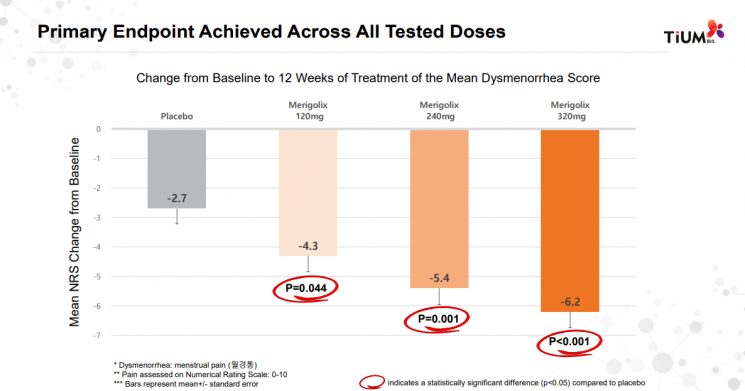 Clinical Phase 2a primary efficacy endpoint results of TiUM Bio's endometriosis treatment 'Marigolicx.' All three dosing groups showed statistically significant efficacy.
Clinical Phase 2a primary efficacy endpoint results of TiUM Bio's endometriosis treatment 'Marigolicx.' All three dosing groups showed statistically significant efficacy. [Image source=TiUM Bio]
The Phase 2a clinical trial results announced by Tium Bio on this day also demonstrated such outcomes. Regarding the primary efficacy endpoint, the reduction of dysmenorrhea, Marigolicx showed statistically significant pain reduction compared to placebo in all dosage groups of 120, 240, and 320 mg. Improvements were also observed in secondary efficacy endpoints, including non-menstrual pelvic pain, dyspareunia, and overall patient pain. Although statistical significance (p-value) was not met in the low-dose group, CEO Kim explained, "Statistical analysis indicated that increasing the number of clinical trial participants would secure statistical significance."
At the briefing, the company emphasized that Marigolicx demonstrated superior efficacy and safety compared to currently used treatments such as Elagolix and Relugolix. CEO Kim stated, "The clinical trials were conducted with the same design as those drugs," adding, "Although it was not a direct comparative trial, there is potential for it to be the best-in-class drug within the category." Regarding one of the major side effects of GnRH antagonists, bone mineral density reduction, there have been no concerns so far. Kim said, "No bone density side effects have appeared up to the 12-week period," and explained, "While such effects may occur with long-term treatment, based on the hormone concentration trends, bone density changes are predicted to be absent. Moreover, if combined hormone therapy is used as with Relugolix, even better results may be achieved."
Tium Bio plans to actively seek partners for Phase 3 clinical trials after receiving the Clinical Study Report (CSR), expected between July and August. CEO Kim said, "We are continuously discussing with several multinational pharmaceutical companies, and through partnering, companies with more resources than us will decide and advance the Phase 3 methods," adding, "As global pharmaceutical companies' interest in women's diseases is increasing, we believe this is the right time for us to enter and expand our business domain."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














