Government "Major Shift in Mental Health Policy"
Experts "Medication Treatment Also Important"
New Treatments Expand Patient Options
Ministry of Health and Welfare "Easing Patient Burden for Long-Acting Injectable Drugs"
 President Yoon Suk-yeol presiding over the Mental Health Policy Vision Declaration Conference at the Blue House Yeongbingwan on the 5th [Image source=Yonhap News]
President Yoon Suk-yeol presiding over the Mental Health Policy Vision Declaration Conference at the Blue House Yeongbingwan on the 5th [Image source=Yonhap News]
President Yoon Suk-yeol has announced that mental health policy will be a national agenda, drawing attention to treatments for mental illnesses.
Mental illnesses occur when the amount of neurotransmitters in the brain is either too high or too low. For example, schizophrenia is caused by excessive dopamine secretion, while depression results from insufficient secretion of serotonin and norepinephrine. Bipolar disorder involves fluctuations in various neurotransmitters, either in excess or deficiency, affecting mood swings.
Experts recommend medication treatment when ▲ mental illness significantly disrupts daily life for more than two weeks, ▲ psychological therapy is ineffective, or ▲ signs of relapse appear. However, many patients discontinue medication due to side effects such as sexual dysfunction and weight gain. Recently, treatments with new mechanisms that reduce side effects have gained attention.
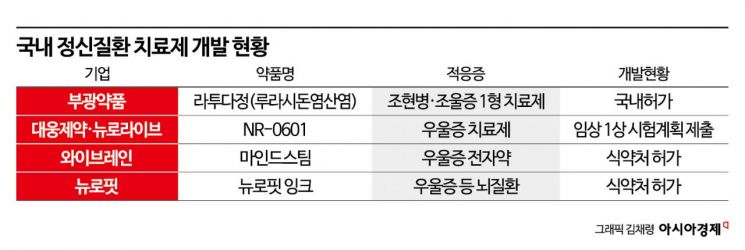
Daewoong Pharmaceutical is on the verge of starting Phase 1 trials for 'NR-0601,' a multi-target-based depression treatment. Developed in collaboration with the biotech company Neurolive, NR-0601 uses electrical signals sent to brain tissue to monitor nerve cell responses, which is expected to reduce side effects compared to existing oral depression medications. It also shows promise for treatment-resistant depression (TRD), which affects about one in three patients with major depressive disorder who do not respond to conventional treatments. The company submitted the Phase 1 clinical trial plan (IND) to the Ministry of Food and Drug Safety last September. A Daewoong Pharmaceutical representative stated, "We expect approval soon and to begin Phase 1 trials shortly."
Last month, Bukwang Pharmaceutical received domestic approval for 'Latuda tablets' (active ingredient lurasidone hydrochloride), a treatment for schizophrenia and bipolar disorder type 1, which reportedly has fewer side effects such as weight gain, dyslipidemia, and hyperglycemia compared to existing treatments. Latuda was developed by the Japanese pharmaceutical company Sumitomo Pharma, and Bukwang Pharmaceutical has held the domestic development and distribution rights since 2017. Bukwang Pharmaceutical completed the application for insurance reimbursement of Latuda tablets in September.
Long-acting injectable medications used for schizophrenia and bipolar disorder differ from daily oral treatments in that they maintain the same effect with injections only once every 3 to 4 months. Although five types of these treatments are available domestically, their relatively high cost has been a burden for patients. In response, the Ministry of Health and Welfare's innovation plan includes measures to reduce out-of-pocket expenses for long-acting injectables. Professor Baek Jong-woo of Kyung Hee University’s Department of Psychiatry commented, "Because long-acting injectables require patient co-payments, economically disadvantaged medical aid patients sometimes discontinued treatment. Including a plan to reduce these co-payments in the government’s mental health policy is a positive development."
Lee Hyung-hoon, Director of Mental Health Policy at the Ministry of Health and Welfare, said, "We will increase the utilization rate of outpatient treatment support programs with no patient co-payments to prevent medication discontinuation cases, such as with long-acting injectables." The outpatient treatment support program provides up to 4.5 million KRW annually for outpatient treatment costs including consultation fees, medication, and tests for severe mental illness patients who have discontinued treatment. Currently, fewer than 100 mental illness patients use this program annually.
For patients who experience side effects or low efficacy from antipsychotics, a new option called 'electroceuticals' has emerged. Ybrain and Neurofit have developed electroceuticals?MindStim and Neurofit Ink, respectively?that treat depression by altering electrical signal transmission in the brain. Both received domestic approval in 2021.
On the 5th, the Ministry of Health and Welfare held a mental health policy vision declaration ceremony chaired by President Yoon Suk-yeol, announcing core tasks for the prevention, early treatment, and recovery of mental illnesses, with a goal to reduce the suicide rate by 50% within 10 years. The number of depression patients surpassed one million for the first time last year, and the number of patients visiting medical institutions for mental illnesses reached 4.11 million in the same year. In the first half of this year, there were 6,936 suicides, an 8.8% increase compared to 6,375 during the same period last year.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















