Securing Domestic Indications for Square Jaw
Researching Hair Loss Indications in High-Dose Development
Focusing on Treatment Indications Overseas
Approval Expected in 2025 with Partner Company’s IPO
Daewoong Pharmaceutical is accelerating the market expansion of its flagship product, the botulinum toxin (BTX) 'Nabota.' Following the successful acquisition of the masseter hypertrophy (square jaw) indication, the company is aiming to extend the dosing interval through higher dosages to enhance dosing convenience. Overseas, it is focusing on targeting the therapeutic market, which holds a larger share.
On the 28th, Daewoong Pharmaceutical announced that Nabota received approval from the Korean Ministry of Food and Drug Safety on the 25th for the indication of masseter hypertrophy (square jaw). This adds the square jaw indication to the existing aesthetic indications of glabellar lines and crow's feet. Notably, this approval is significant as it is the first time among global BTX products that indications for both upper and lower facial major treatment areas have been simultaneously secured.
Nabota is one of the key products driving Daewoong Pharmaceutical's recent performance. In the United States, where it is marketed under the brand name 'Jeuveau,' it has shown an average annual sales growth rate of 62% over two years, surpassing a 10% market share. It is especially popular among the MZ generation, increasing its potential for sustained growth. In Europe, marketed as 'Nuceiva,' its influence is expanding rapidly with launches in the UK, Germany, Austria, and now Italy.
Extending Dosing Intervals... 'Nabota' Eyes Hair Loss Treatment
Daewoong Pharmaceutical is focusing on various research and development (R&D) efforts to sustain growth. It is working on diversifying dosing intervals through the development of high-dose units and concentrating on expanding therapeutic indications through its overseas partner, Eon Bio Pharma.
Daewoong’s overseas aesthetic partner, Evolus, successfully completed a Phase 2 clinical trial last month to demonstrate the long-term efficacy of Jeuveau over six months. The trial involved administering a high dose of 40 units to over 150 patients under 65 years old with moderate to severe glabellar lines for 12 months, confirming a long-lasting effect of approximately six months. In terms of safety, no serious adverse effects were observed, and the side effect profile was similar to existing BTX products and Jeuveau’s standard doses.
Currently, most developed BTX products require injections every 3 to 4 months. Doubling the dosing interval is expected to improve dosing convenience and enhance competitiveness in customer acquisition. Additionally, the extended dosing interval is anticipated to help prevent the development of resistance in the body, which is one of the drawbacks of BTX. Last year, the US FDA approved 'Daxxify' by Revance Therapeutics, which extended the dosing interval to 6 to 9 months.
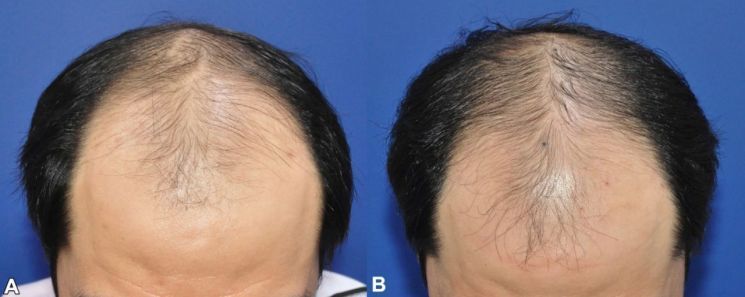 Researcher's Visual Assessment Before and After Botulinum Toxin 'Nabota' Injection in Male Pattern Baldness Areas
Researcher's Visual Assessment Before and After Botulinum Toxin 'Nabota' Injection in Male Pattern Baldness Areas [Photo by Daewoong Pharmaceutical]
Moreover, Daewoong Pharmaceutical is exploring the potential of Nabota for hair loss indications, announcing research results showing a statistically significant increase in hair count at 24 weeks after direct scalp administration in male pattern hair loss patients. In the US, Allergan is conducting clinical trials to verify the efficacy of the BTX product 'Xeomin' for androgenic alopecia treatment.
Therapeutic BTX Accounts for Half of Global Market... Development Accelerated by Partner’s IPO
Daewoong Pharmaceutical is also making efforts to pioneer the therapeutic market for Nabota. Originally, BTX was used primarily for therapeutic purposes such as eyelid spasms before its wrinkle-improving effects were discovered, leading to expanded aesthetic indications. Although 90% of the domestic BTX market is estimated to be for aesthetic use, according to market research firm FBI, the global BTX market size last year was $6.5 billion (approximately 8.6 trillion KRW), with the therapeutic market accounting for $3.44 billion (approximately 4.546 trillion KRW), representing a majority share of 53.3%. With the therapeutic market expected to continue growing, it is considered to have significant potential as the domestic market has yet to fully open.
Development of therapeutic indications is mainly conducted through the overseas therapeutic partner, Eon Bio Pharma. Clinical trials are underway for indications including cervical dystonia, chronic and episodic migraine, gastroparesis, and post-traumatic stress disorder (PTSD).
The most advanced is cervical dystonia. Cervical dystonia is a condition where neck muscles contract involuntarily, causing abnormal neck postures. In the previously announced Phase 2 topline results, all dosage groups of Nabota?low, medium, and high?showed significant improvement compared to placebo on the primary endpoint, the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS). A Phase 3 trial is planned to begin next year.
A Phase 2 trial for chronic and episodic migraine is also underway. Notably, episodic migraine is an indication that no existing BTX product has secured. Topline results for episodic migraine are expected this fall, with chronic migraine results to follow next year.
With Eon Bio Pharma recently successfully listing on NASDAQ via a SPAC, securing $125 million (approximately 165.2 billion KRW) in funding, the development of therapeutic indications is expected to gain further momentum. Researcher Park Jong-hyun from Daol Investment & Securities commented, "Approval of therapeutic toxins in the US is anticipated by 2025," adding, "Eon Bio Pharma’s listing highlights the commercial potential of therapeutic toxins." Daewoong Pharmaceutical and the Daewoong Group hold a 21.4% stake in Eon Bio Pharma.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














