Successful Phase 2 Clinical Trial with Double the Capacity Compared to Previous
Daewoong Pharmaceutical announced on the 4th that its botulinum toxin (BTX) product 'Nabota' (U.S. product name: Jeuveau) has successfully completed a high-dose Phase 2 clinical trial in the U.S. to demonstrate long-lasting effects for 6 months in patients with glabellar wrinkles.
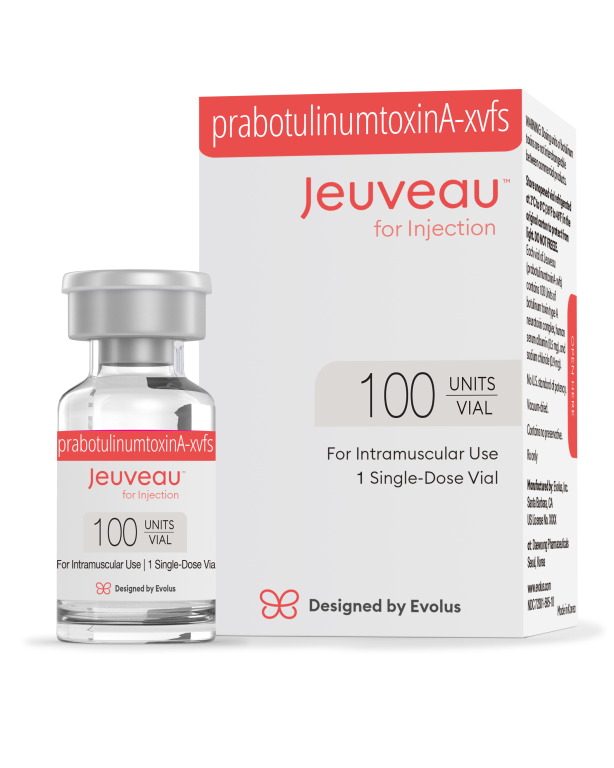 Daewoong Pharmaceutical's botulinum toxin product 'Juvo' sold locally in the United States.
Daewoong Pharmaceutical's botulinum toxin product 'Juvo' sold locally in the United States. [Photo by Daewoong Pharmaceutical]
This clinical trial was conducted by Daewoong Pharmaceutical's U.S. partner Evolus, involving 150 patients under 65 years old with moderate to severe glabellar wrinkles. The study was a multicenter, double-blind, randomized trial carried out over 12 months starting from March last year.
Daewoong Pharmaceutical stated that the study confirmed the long-lasting effect of 6 months or 26 weeks with a high dose of 40 units, while also demonstrating safety without serious adverse effects. The active control groups for the 40-unit Jeuveau were the original products of 20 units of Botox and 20 units of Jeuveau, and the adverse event results were similar to those of the active control groups.
Daewoong Pharmaceutical emphasized that through this clinical trial, the high-dose Jeuveau secures flexibility in treatment cycles, enabling various options. They further explained that treatment cycles longer than 6 months are options desired by many customers in the global market. Most botulinum toxin products sold worldwide have an effect duration of around 3 months.
Daewoong Pharmaceutical and Evolus plan to expand their market share in the global botulinum toxin market by offering various treatment cycle options to enhance competitiveness. Park Sung-soo, Vice President of Daewoong Pharmaceutical, said, "The successful completion of the high-dose administration trial by our partner Evolus is very encouraging. Based on the meaningful results derived from this trial, we plan to conduct marketing in major botulinum toxin markets such as the U.S., Europe, Canada, and Australia." He added, "Both companies will continue clinical trials to prove the efficacy and safety of Nabota and expand its global market share."
Meanwhile, Evolus announced interim results of the 'Extra Strength' Phase 2 clinical trial at the International Society of Aesthetic Plastic Surgery held in Paris, France, last January. The trial involved administering a high dose of 40 units of Jeuveau, approximately double the existing 20-unit dose, and observing the improvement of glabellar wrinkles over 6 months. Evolus plans to present the final results of the one-year clinical trial at the U.S. Medical Conference in the fourth quarter.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















