Institute for Basic Science Observes Reactive Astrocyte Changes
Acetate Metabolism Decline Captured by PET Imaging
Domestic researchers have developed an imaging technology that can observe how brain cells change in patients with Alzheimer's dementia. This brings a positive signal for the early diagnosis and treatment of dementia, which is the greatest challenge to health and welfare in the super-aged society.
The Institute for Basic Science (IBS) announced on the 17th that the research team led by Director Changjun Lee of the Center for Cognition and Sociality, together with Professor Mijin Yoon of the Department of Nuclear Medicine at Severance Hospital and Principal Researcher Hoon Ryu of the Brain Science Institute at the Korea Institute of Science and Technology (KIST), succeeded in visualizing reactive astrocytes and the resulting neuronal metabolic decline in the brains of Alzheimer's dementia patients.
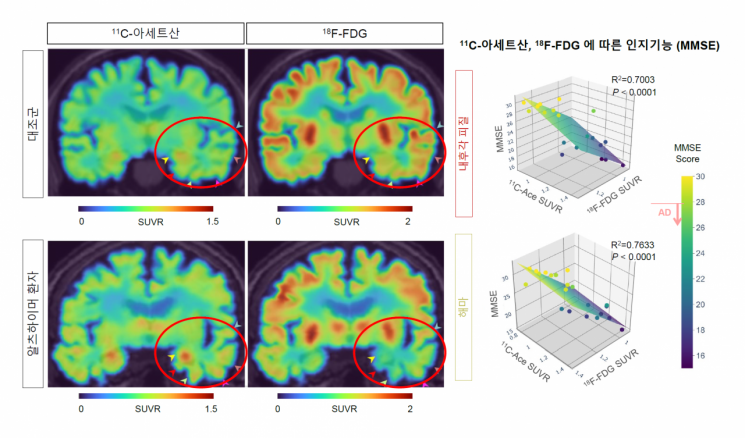 Correlation between cognitive function and changes in PET imaging and 11C-acetate and 18F-FDG uptake in Alzheimer's patients. Image source provided by IBS.
Correlation between cognitive function and changes in PET imaging and 11C-acetate and 18F-FDG uptake in Alzheimer's patients. Image source provided by IBS.
Alzheimer's dementia, one of the representative causes of senile dementia, is well known to involve brain inflammatory responses. One of the earliest phenomena during brain inflammation is reactive astrogliosis, where the size and function of astrocytes?star-shaped non-neuronal cells that constitute the majority of brain cells?change.
In previous studies, the research team confirmed that reactive astrocytes express the enzyme monoamine oxidase B (MAO-B) and produce the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) from putrescine, leading to memory decline. Recently, they identified the presence of the urea cycle within astrocytes and demonstrated that the activated urea cycle promotes dementia.
However, despite the clinical importance of reactive astrocytes, brain neuroimaging technology capable of meaningfully visualizing these cells at the clinical level for observation and diagnosis has not yet been developed.
The research team demonstrated that positron emission tomography (PET) imaging using Carbon-11 acetate (11C-acetate) and Fluorine-18 fluorodeoxyglucose (18F-FDG) can visualize reactive astrocytes and the associated neuronal glucose metabolic decline in Alzheimer's patients. PET is a technology that measures positrons emitted by radiopharmaceuticals selectively binding to specific substances, providing three-dimensional physiological, chemical, and functional images of the human body. 11C-acetate images cells that absorb acetate, primarily used in cancer diagnosis. 18F-FDG tracks glucose to monitor brain activity.
Through PET imaging of an animal model inducing reactive astrocytes, the researchers revealed that reactive astrogliosis activates acetate metabolism in reactive astrocytes and induces suppression of glucose metabolism in surrounding neurons. Comprehensive analyses including immunohistochemistry and electrophysiological methods showed that acetate promotes reactive astrogliosis, leading to the production of putrescine and GABA, thereby causing dementia.
Acetate, commonly known as "vinegar," functions as an energy source for astrocytes. It is excessively absorbed by reactive astrocytes via monocarboxylate transporter 1 (MCT1), which is specifically expressed in astrocytes, further promoting reactive astrogliosis. Additionally, acetate was found to enhance reactive astrogliosis, urea cycle activation, and subsequent putrescine and GABA production in astrocytes treated with the toxic substance amyloid beta.
Conversely, when reactive astrogliosis or MCT1 expression was inhibited, acetate metabolism in astrocytes and glucose metabolism in surrounding neurons were restored to normal. These metabolic changes were consistently observed in various Alzheimer's animal models and actual Alzheimer's patients, with more severe metabolic alterations correlating with greater cognitive decline in patients.
Until now, amyloid beta has been known as the main cause of dementia. However, PET imaging targeting amyloid beta has limitations in clinical diagnosis of patients. Furthermore, all dementia drugs aimed at removing amyloid beta have failed to date.
This study demonstrated the potential of PET imaging using 11C-acetate and 18F-FDG to clinically diagnose reactive astrocytes and functionally suppressed neurons, showing promise for early diagnosis of Alzheimer's dementia. Most importantly, by elucidating the mechanism by which acetate and the MCT1 transporter promote reactive astrogliosis, the study proposed a new target for dementia treatment.
The research team stated, "This study shows that reactive astrocytes, recently highlighted as a core cause of dementia, can be directly visualized in patient brains, which has significant academic and clinical value," and added, "We revealed that acetate not only serves as an energy source for astrocytes but also promotes reactive astrogliosis, presenting a new mechanism by which reactive astrogliosis is induced in brain diseases."
They continued, "Reactive astrocytes exhibited abnormal metabolism by excessively absorbing acetate, unlike normal conditions. We discovered that this absorbed acetate plays a crucial role in promoting intracellular inflammatory responses," and said, "Through Alzheimer's animal models, we confirmed significant recovery when inhibiting MCT1, the pathway for acetate transport. MCT1 could be a new therapeutic target for Alzheimer's dementia."
The research results were published online on the 17th in the neuroscience journal Brain (IF=15.255).
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














