'1st Medical Device Industry Promotion and Support Comprehensive Plan'
Focus on Investment in New Technologies such as Digital Health
Support for Securing Clinical Evidence and Breakthroughs in Overseas Approvals
 Last month, visitors toured the exhibition hall at the 38th International Medical Devices and Hospital Equipment Exhibition (KIMES 2023) held at COEX in Samseong-dong, Gangnam-gu, Seoul.
Last month, visitors toured the exhibition hall at the 38th International Medical Devices and Hospital Equipment Exhibition (KIMES 2023) held at COEX in Samseong-dong, Gangnam-gu, Seoul. [Image source=Yonhap News]
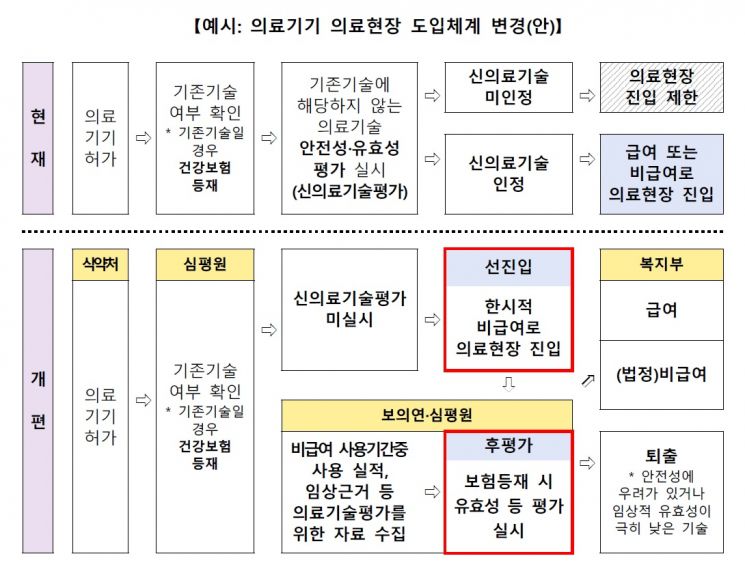
Up to 10 trillion KRW will be invested in research and development (R&D) of the medical device industry over the next five years. Until now, even if innovative medical devices were developed and received approval, they often faced difficulties in the commercialization process. However, going forward, these devices will be able to enter the market as non-reimbursable immediately upon approval, and a "pre-market entry, post-market evaluation" system will be established to assess products through future health technology assessments.
The Ministry of Health and Welfare announced the "1st Comprehensive Plan for Fostering and Supporting the Medical Device Industry ('23~'27)" on the 4th, which includes these details. This comprehensive plan is the first mid- to long-term statutory plan established under the "Medical Device Industry Promotion and Innovative Medical Device Support Act," which came into effect in May 2020. It will be formulated every five years going forward.
First, the government proposed expanding the scale of R&D investment by both the private sector and government to 10 trillion KRW. Investments will be concentrated in key export sectors, high-potential fields, and public sectors. The ongoing "Pan-Governmental Full-Cycle Medical Device R&D" project, currently running until 2025, will be planned through a second phase to continuously promote government-led R&D support. In particular, significant investments are expected in promising fields such as digital health, medical robots, and implantable devices. In the digital health sector, products integrating artificial intelligence (AI) and big data for disease prevention, diagnosis, and treatment, as well as digital therapeutics (DTx), will be developed, and service model development utilizing these technologies will be supported.
The development of the medical device industry will also respond to social changes. In response to aging, R&D investments will increase in assistive and rehabilitation devices and care robots, while supporting the development of electronic medicines capable of treating dementia and chronic diseases. Mobile medical platforms will be supported for disaster and emergency sites, which are medical blind spots, and localization of essential medical devices, which are highly dependent on imports but necessary for treating rare and intractable diseases, will be promoted.
To activate the use of domestic medical devices, a clinical validation support project will be conducted based on the judgment that despite the presence of excellent domestic products, their use is low in major domestic general hospitals due to lack of user experience, accuracy, reliability, and performance degradation concerns. To this end, large-scale validation infrastructure will be established and operated. The currently operating Innovative Medical Device Validation Support Center will be expanded, setting eight key fostering fields such as digital health and medical robots, and establishing a mid- to long-term validation support system equipped with overseas regulatory response capabilities. The user evaluation project, which allows medical personnel to directly use and evaluate domestic medical devices, will be expanded, and a purchase voucher program will be implemented for innovative medical devices to promote wider use of products with confirmed performance.
For global expansion, tailored strategic roadmaps will be established by region, and overseas cooperation governance will be built. Considering that the standards for approval, the first gateway, are increasingly stringent overseas, a "MedTech Export Support Task Force" will be formed, and breakthroughs will be sought through joint research and clinical support with overseas medical institutions and companies.
In particular, considering criticism that innovative medical technologies often fail to properly enter the market despite being developed through such processes, active efforts will be made to rationalize market entry regulations for innovative technologies and foster the ecosystem. First, in terms of regulation, a plan will be devised to allow safety and efficacy results evaluated during medical device approval to be utilized in new medical technology assessments and health insurance listings. Regulatory rationalization will be pursued to facilitate smooth approval, including temporary product classification for new technologies and simplification of clinical trial approvals.
At the market entry stage, in the short term, expansion of the integrated review and evaluation system for innovative medical devices and the scope of application for the new medical technology assessment deferral system will be considered. In the mid- to long term, a "pre-market entry, post-market evaluation" system will be introduced. For non-invasive innovative medical devices such as DTx, which have no safety concerns and are not in essential medical areas, market entry will be allowed immediately as temporary non-reimbursable once approval is obtained, without undergoing new medical technology assessment. Through this, real-world data (RWD) will be secured to enable evaluation of efficacy and other factors necessary for actual insurance listing. Health technology assessment will be converted into a procedure for health insurance application, recognizing health insurance reimbursement or maintaining non-reimbursable status, and if problems arise, allowing market withdrawal.
Jung Eun-young, Director of the Health Industry Policy Bureau at the Ministry of Health and Welfare, explained, "We will significantly expand the integrated review of innovative medical devices and the new medical technology assessment deferral system. From 2025, the Ministry of Food and Drug Safety will allow more clinical data to be reviewed during approval, and once approved, devices will be introduced to the market as non-reimbursable, with a system linking new medical technology assessment accordingly."
Cho Kyu-hong, Minister of Health and Welfare, said, "Following the growth of our medical device industry triggered by COVID-19, it is time to establish mid- to long-term support strategies to sustain growth and maintain export momentum. Through the first mid- to long-term statutory comprehensive plan, we will strengthen cooperation with the industry and do our best to secure budgets so that our medical device industry can gain global competitiveness and lead the bio-health industry."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














