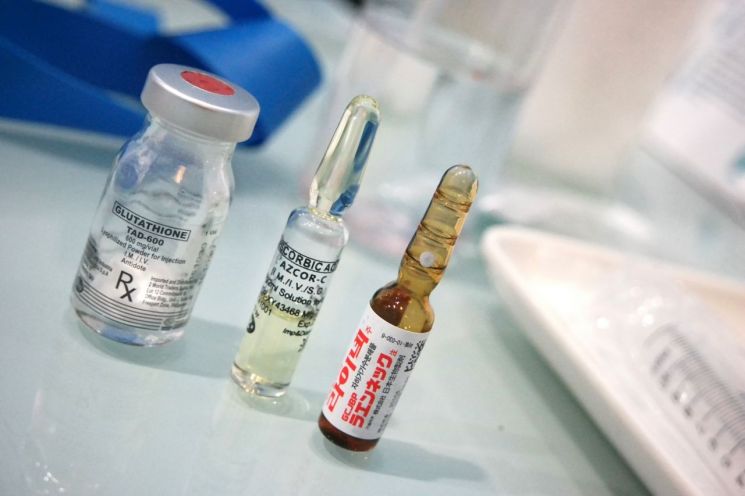
[Asia Economy Reporter Seonjin Byun] Starting in April, the provision of pharmaceutical package inserts in the form of QR codes instead of paper will be allowed for certain medicines. This is the first time that package inserts, which inform users about side effects and risks when using medicines, will be provided as electronic information.
From April, package inserts for injectable drugs administered directly will be provided via QR codes... Industry Cheers
The Ministry of Food and Drug Safety (MFDS) announced at the ‘100 Major Tasks for Regulatory Innovation in Food and Drug’ performance report meeting held on the 2nd that it will implement the phased introduction of pharmaceutical e-labels and conduct a pilot project from April this year through next year. Initially, package inserts for injectable prescription drugs administered directly at medical institutions will be provided experimentally via QR codes. Since pharmaceutical package inserts must contain a vast amount of drug information on limited paper space, there have been criticisms about small font sizes leading to poor readability. Additionally, when information such as drug approval details changes, the package insert content must be updated within at least 1 to 3 months, which was a disadvantage.
The pharmaceutical industry is immediately welcoming this move. Hyeryeon Bang, Executive Director at Korea AstraZeneca, said, “The pharmaceutical information for anticancer drugs is at the level of a booklet, and diabetes medications contain over 40 to 50 pages of extensive drug information, which was inefficient. Also, patients generally do not check the information on injectable drugs, which was another drawback.” She added, “Korea’s infrastructure is the optimal environment for introducing pharmaceutical e-labels. I hope this will be applied and utilized for various prescription drugs in the future.” Bang also expressed hope that “starting with QR codes, electronic package inserts using blockchain technology will also emerge.”
The MFDS is considering unifying the provision of package inserts for all medicines in electronic format, starting with injectable drugs administered directly at medical institutions. Seokyeon Kang, Director of the Pharmaceutical Division at MFDS, said, “Providing drug information through mobile devices and other means is expected to reduce manufacturing and printing costs for pharmaceutical companies. It will also enable healthcare professionals and patients to receive the latest information quickly and efficiently in real time.”
MFDS: “Out of 50 pharmaceutical tasks, 23 completed or institutionalized”
 The Ministry of Food and Drug Safety held the '100 Major Tasks for Regulatory Innovation in Food and Medicine Progress Report Meeting' on the 2nd. Photo by Seonjin Byun sj@
The Ministry of Food and Drug Safety held the '100 Major Tasks for Regulatory Innovation in Food and Medicine Progress Report Meeting' on the 2nd. Photo by Seonjin Byun sj@
In August last year, the MFDS established the ‘100 Major Tasks for Regulatory Innovation in Food and Drug’ aimed at becoming a ‘Global Hub for Bio and Digital Health.’ On this day, it explained that out of 50 pharmaceutical-related tasks, 23 have been completed or institutionalized.
The introduction of the ‘Global Innovative Fast Track (GIFT)’ system was implemented in September last year. GIFT is a system that shortens the review period and pre-examines prepared data to enable global innovative products to be commercialized quickly. ‘Lunsumio Injection,’ a treatment for relapsed or refractory follicular lymphoma developed by Roche Korea, was designated as the first target under this system in November last year, expanding treatment opportunities for patients with rare and intractable diseases.
The ‘Customized Fast Classification System’ for new industry medical devices such as digital health devices was also introduced. This system temporarily classifies new and convergent medical devices to allow approval and certification. Since new medical devices in emerging fields do not fall under the MFDS’s existing product classifications, they had to apply for approval under similar subcategories, which caused long delays in commercialization. The MFDS explained that five advanced technology software medical devices were designated as fast classification items on December 29 last year. Accordingly, medical devices for ▲skin cancer image detection and diagnostic support ▲speech and language disorder diagnostic support ▲eye movement analysis ▲musculoskeletal rehabilitation ▲bladder cancer image detection and diagnostic support can now prepare clinical and approval data early without classification procedures, shortening the process by a total of 60 days.
Additionally, the pharmaceutical task of ‘expanding the designation targets for innovative medical devices’ has been applied since October last year, and the number of additional designations for innovative medical devices increased by 37% to 26 cases compared to 19 cases before implementation. Previously, when evaluating innovative medical device designations, the characteristics of the four classified groups were not considered, and common evaluations were made based on technological innovation, safety, efficacy improvement, industrial value, and public interest value. With the new system, evaluations focus on key items specific to each group. Designation as an innovative medical device allows priority review, reducing the approval period from an average of 80 days to 40 days.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














