Nearly 2,400 COVID-19 Clinical Trials Worldwide
9 Trials Including Remdesivir Underway in Korea... Struggling Due to Declining Patients
[Asia Economy Reporter Choi Daeyeol] Korean pharmaceutical company GC Green Cross began developing a vaccine in 2005 when avian influenza spread. They invested 10 billion KRW over 10 years to develop and obtain approval for the vaccine. However, production was never carried out as the outbreak ended. Yu Hyun-ah, head of the GC Green Cross research institute, said, "Vaccines require continuous production and data management after development, but we failed to do so," adding, "Although there was some government support at the time, the development costs were not recovered."
As COVID-19 patients increase, governments and private companies worldwide are engaging in vaccine and treatment development. While all experts agree that vaccines and treatments are necessary to end the pandemic, there are already forecasts that development will not be easy. This is because clinical trials are difficult to conduct as patient numbers decline.
Domestic Clinical Trials Struggle to Get Started
Only 25 Severe Patients in Korea
Clinical Plans Approved but Progress Stalled
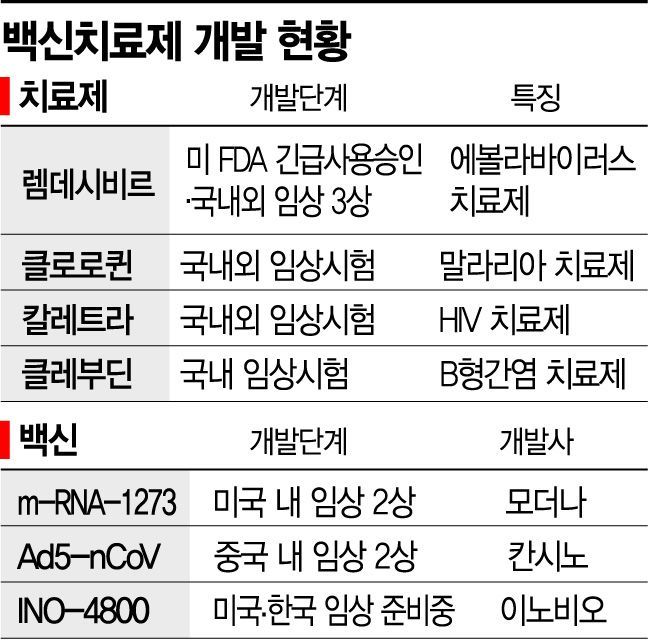
According to the World Health Organization (WHO), there are a total of 2,371 clinical trials related to COVID-19 treatments and vaccines ongoing worldwide (as of the 12th, including investigator-initiated trials). In Korea, 9 clinical trials focusing on treatments are underway. Drug repositioning, which tests whether drugs previously developed or under development for other diseases?such as remdesivir for Ebola virus, chloroquine for malaria, and levovir for hepatitis B?are effective against COVID-19, is progressing rapidly.
The Korean government has stated that a COVID-19 treatment could be available domestically as early as the end of this year, which is also a case of drug repositioning. New drug development requires examining the efficacy of the substance on cells or animals, followed by thorough safety, efficacy, and side effect evaluations in humans. In the case of repositioning, toxicity and harmfulness have already been verified, allowing for faster progress.
 Minister of Health and Welfare Park Neung-hoo visited GC Green Cross in Yongin-si, Gyeonggi-do, which is developing a plasma treatment for COVID-19, on the afternoon of the 13th to inspect the research facilities.
Minister of Health and Welfare Park Neung-hoo visited GC Green Cross in Yongin-si, Gyeonggi-do, which is developing a plasma treatment for COVID-19, on the afternoon of the 13th to inspect the research facilities. Recently approved remdesivir in the US and Japan is also undergoing clinical trials in Korea but faces difficulties. Three clinical trial plans were approved in early March; only one has completed patient recruitment, while two remain in the recruitment phase. According to the clinical plans disclosed by companies, clinical trials targeting severe patients require 98 participants in Korea, but currently, there are only 25 patients at severe stages or above (as of the 12th).
Among approximately 1,000 isolated and treated patients, most have mild or no symptoms or have recovered. While efforts in quarantine and treatment are yielding results, new drug development faces paradoxical difficulties. Professor Kim Woo-joo of the Department of Infectious Diseases at Korea University Guro Hospital said, "It takes too long to get clinical plans reviewed by each institution," adding, "Even if trial approval is obtained, it has become difficult to conduct trials due to the decreasing number of patients."
US and China Enter Phase 2 Trials... Aim for Commercialization Next Year
Joint Clinical Trials with US Company to Begin Next Month in Korea
Declared 'Stockpiling' but No News for a Month
Vaccine development is also challenging. The fastest progress is seen in US-based Moderna and China’s CanSino. Both have entered Phase 2 clinical trials domestically and aim for commercialization within next year. In Korea, the National Institute of Health, US company Inovio, and the International Vaccine Institute have agreed to conduct joint clinical trials. They plan to start trials as early as next month, targeting a launch in the second half of next year. Korean vaccine company SK Bioscience is currently identifying candidate substances and testing their efficacy in animals. The company expects to begin clinical trials from September.
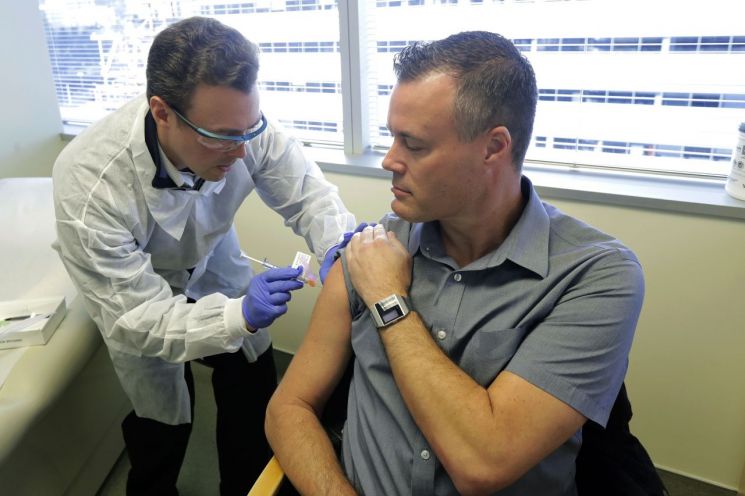 At the Kaiser Permanente Washington Health Research Institute in Seattle, USA, on the 16th (local time), a pharmacist is administering a COVID-19 vaccine candidate to a participant in a Phase 1 clinical trial.
At the Kaiser Permanente Washington Health Research Institute in Seattle, USA, on the 16th (local time), a pharmacist is administering a COVID-19 vaccine candidate to a participant in a Phase 1 clinical trial. However, industry insiders and researchers emphasize the need for practical government support rather than premature optimistic forecasts. President Moon Jae-in encouraged the development of COVID-19 treatments and vaccines and expressed intentions to stockpile them on April 9, but it is reported that no stockpiling plan has been finalized even after a month.
Yu Hyun-ah said, "In advanced countries like the US and Europe, partnerships between public research institutes and pharmaceutical companies are formed to respond during crises, and stockpiling after development is also promised," adding, "This is not an issue that individual countries or companies can handle alone, and since it is difficult to overcome through competition, it is necessary to lead cooperation between governments, the private sector, and even between countries."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














