Professor Jung Kyungmin's Team Develops High-Output, High-Capacity Electrodes Through Internal Pore Structure Design
Theoretical Correlation Between Pore Structure and Battery Performance Proposed, Published in Adv. Energy Mater.
When the thickness of battery electrodes is increased to boost capacity, a drop in output performance typically occurs. However, a new thick-film electrode has now been developed to address this issue.
This signals a breakthrough for electric vehicles that can maintain power even when climbing hills, despite having longer driving ranges.
On January 5, a team led by Professor Jung Kyungmin from the Department of Energy and Chemical Engineering at UNIST announced the development of a high-capacity electrode that increases output by 75% compared to conventional electrodes by optimizing the porous structure within thick-film battery electrodes.
 Research team, Professor Jung Kyungmin (left), Researcher Jeon Byungjin (first author). Provided by UNIST
Research team, Professor Jung Kyungmin (left), Researcher Jeon Byungjin (first author). Provided by UNIST
Driving range is undoubtedly the central topic in the electric vehicle market. This is why thick-film electrode technology, which increases battery capacity by stacking thicker electrodes, is gaining attention. However, as electrodes become thicker, their instantaneous power output decreases. This is because the distance lithium ions must travel increases with electrode thickness, and the pathways become more complex, slowing down the discharge process.
The electrode developed by the research team delivers high output performance, even with a high areal capacity of 10 mAh/cm². In particular, under a high-output 2C environment, conventional electrodes achieved an areal capacity of only 0.98 mAh/cm², whereas the newly developed electrode reached 1.71 mAh/cm². This means the amount of electric energy that can be extracted in a short period increased by about 75%.
The research team developed this electrode based on an analytical method that classifies pores within the electrode into two types. Inside the electrode, there are large pores between particles that allow lithium ions to pass through relatively easily, and there are micro-pores (CBD structure) formed by the aggregation of conductive additives and binders. The team determined that these micro-pores hinder the flow of lithium ions.
To analyze this quantitatively, the team developed and utilized an in-house Dual-Pore Transmission Line Model (DTLM). Based on this quantitative analysis, they optimized the internal structure of the electrode by adjusting the manufacturing process and the content of conductive additives.
Jeon Byungjin, the first author of the study, stated, "The equations derived from our quantitative analysis will serve as an important foundation for applying Physics-Informed Neural Network (PINN) technology, which learns based on formulas when data is scarce, to battery design in earnest."
Professor Jung Kyungmin explained, "In the era of thick-film electrodes, it will be important not only to focus on the intrinsic properties of materials but also to enhance the utilization of the microstructures they form. This research will play a significant role not only in high-nickel batteries but also in the development of next-generation batteries such as lithium iron phosphate (LFP) batteries, which are challenging to design due to their high proportion of conductive additives."
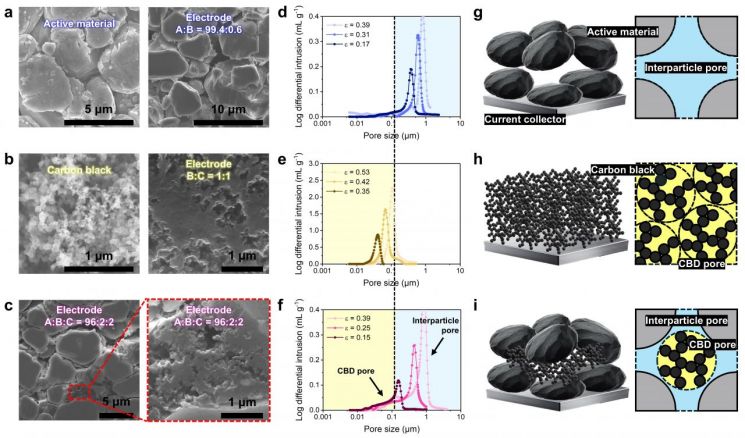 Different microstructures and pore structures observed in binary and ternary composition electrodes.
Different microstructures and pore structures observed in binary and ternary composition electrodes.
This study was published on December 12 in Advanced Energy Materials, an international journal in the field of energy and environment.
The research was carried out as part of the "Development of Continuous Large-Area Manufacturing Equipment for Dry Electrodes for Secondary Batteries" project, conducted by the Korea Evaluation Institute of Industrial Technology (KEIT) with support from the Ministry of Trade, Industry and Energy (MOTIE).
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
















