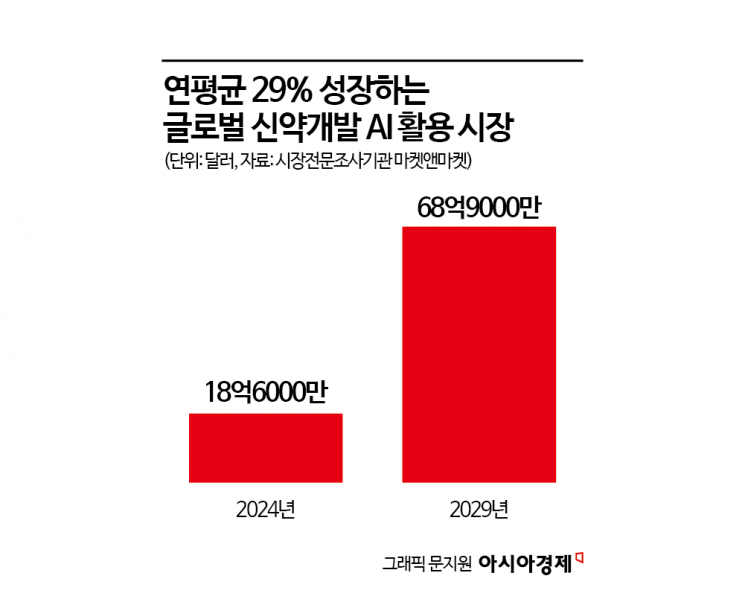
AI-Driven Drug Development Market Grows at 29% Annually
Building Industrial Infrastructure: Data, Regulation, and Talent Needed
In the global pharmaceutical and biotech industry, utilizing artificial intelligence (AI) for new drug development has become a necessary condition for transforming the cost and time structure of the process. However, in South Korea, the field remains at the stage of technological validation, revealing structural limitations. This has led to calls for building industrial infrastructure centered on data, regulations, and talent.
According to the policy report "Industrialization Strategy for AI-Based New Drug Development," published by the Korea Biotechnology Industry Organization on December 15, traditional new drug development typically takes 10 to 15 years, requires an average investment of 1 to 2 trillion won, and results in only one out of 10,000 candidate compounds (less than 0.01%) reaching the market, highlighting an entrenched low-efficiency structure.
As a result, the global market for AI-driven new drug development is projected to grow from 1.86 billion dollars (approximately 2.7384 trillion won) last year to 6.89 billion dollars (about 10.1441 trillion won) by 2029, with an average annual growth rate of 29.9%, according to market research firm MarketsandMarkets. Major countries are also simultaneously advancing both policy and infrastructure in this field.
The United States has proposed investments of around 700 trillion won in AI infrastructure, including data centers, semiconductors, and cloud computing, and is accelerating AI adoption through the Food and Drug Administration (FDA) by launching pilot programs for AI-based drug review and evaluation and issuing related guidelines. China, in its "Pharmaceutical Industry Digital Transformation Promotion Plan (2025-2030)," has officially designated AI-driven drug development as a top priority.
In the private sector, Isomorphic Labs, Alphabet's AI drug development subsidiary, has signed joint development agreements with Eli Lilly (1.7 billion dollars) and Novartis (1.2 billion dollars). Insilico Medicine is emphasizing a compressed development cycle, highlighting a process of 21 days for design and 46 days for synthesis and preclinical validation-15 times faster than the conventional 2 to 3 years. Chinese biotech companies have also achieved results this year by signing technology transfer deals with major pharmaceutical companies for AI-driven drug candidates, amounting to tens of trillions of won.
The report suggests that, to substantially increase the use of AI in South Korea's pharmaceutical and biotech industry, it is essential to establish industrial infrastructure-namely, data, regulations, and talent-before focusing on individual companies' algorithmic competitiveness. On the data front, the report recommends simplifying procedures for combining and analyzing pseudonymized information under strict security conditions, and expanding regulatory sandboxes within government-designated "Safe Zones" to facilitate smoother data integration and analysis.
 Daewoong Pharmaceutical researchers are exploring new drug candidate compounds through an AI-based new drug development system. Daewoong Pharmaceutical
Daewoong Pharmaceutical researchers are exploring new drug candidate compounds through an AI-based new drug development system. Daewoong Pharmaceutical
The report also mentions the consideration of a "data utilization immunity provision" to mitigate liability in the event of unintentional incidents. Regarding regulations, it points out a bottleneck in the lack of clear guidelines on how to trust and recognize AI-derived results at the Investigational New Drug (IND) application stage. The report recommends establishing Good Machine Learning Practice (GMLP) standards that encompass the development, validation, and operation of machine learning models, and clarifying data sources and quality, model design, performance evaluation metrics, and reproducibility verification methods.
The report also proposes a recognition roadmap for in-silico data, initially limiting its use to supplementary material for toxicity prediction and drug-target binding prediction, but gradually expanding its acceptance to replace certain experimental stages as accuracy and reproducibility are verified. In-silico data refers to new drug development data generated through computer simulation, modeling, or AI prediction.
On the talent front, the report emphasizes the need to foster "bilingual" interdisciplinary professionals who can understand and utilize both AI and biotechnology at the national level. It recommends making AI and data science mandatory in medical, pharmaceutical, and life science curricula; strengthening new drug development tracks and project-based learning (PBL) in AI graduate schools; and cultivating problem-solving professionals through rotational and dispatch programs among pharmaceutical companies, AI startups, universities, and hospitals.
The report states, "Although domestic companies are already expanding the scope of AI utilization to candidate discovery, integrated R&D platforms, literature search, data analysis, and automated document preparation, for these trends to translate into industrial competitiveness, data must flow freely and evaluation criteria must be clear," adding, "The three key elements-a seamless data environment, clear regulatory standards, and interdisciplinary teams with field experience-must be established simultaneously."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.