UNIST Identifies Mechanism by Which AIM2 Protein Triggers Inflammatory Cell Death During Mpox Infection
Lays Groundwork for Therapeutic Development Against Severe Viral Infections... Published in Cell Mol Immunol.
A team of Korean researchers has identified a protein sensor that acts as a "trigger" for the progression of severe Mpox (monkeypox).
This protein was found to recognize the DNA of the Mpox virus that has invaded the body, thereby inducing a strong inflammatory response.
On November 25, Professor Lee Sangjun's team from the Department of Biological Sciences at UNIST, in collaboration with Director Kim Yujin from the Korea National Institute of Health and Professor Kim Daesik's team from Sungkyunkwan University School of Medicine, announced that they have experimentally demonstrated that a protein called AIM2 is a major cause of excessive inflammatory responses during Mpox infection.
 Research team (from left) Professor Lee Sangjun, Researcher Oh Jueun (first author), Researcher Lee Jihye (first author). Provided by UNIST
Research team (from left) Professor Lee Sangjun, Researcher Oh Jueun (first author), Researcher Lee Jihye (first author). Provided by UNIST
So far, the reported fatality rate of Mpox is around 3%, which is not particularly high. However, the situation changes if an excessive inflammatory response occurs within the body. While inflammation is a normal response by the immune system to eliminate viruses, if it becomes too intense, it can destroy healthy tissue and actually worsen the disease.
Even healthy young people can lose their lives to influenza or COVID-19 due to an uncontrolled inflammatory response known as a "cytokine storm."
According to the research findings, AIM2 acts as a "sensor" that recognizes the DNA of the invading Mpox virus. Once activated by recognizing viral DNA, AIM2 forms an inflammasome, which in turn activates an enzyme called caspase-1. This process leads to cell destruction and the simultaneous secretion of inflammatory signaling molecules (IL-1β, IL-18).
AIM2 was found to affect not only cells directly infected by the virus but also surrounding uninfected cells, causing tissue-wide inflammatory damage and contributing to disease severity. In the experiments, pyroptosis was observed in directly infected cells, while apoptosis and necroptosis-other forms of cell death-were observed in surrounding cells. Depending on the intensity and progression of the inflammatory response, cell death can be classified as highly inflammatory pyroptosis, moderately inflammatory necroptosis, or apoptosis, which is accompanied by little to no inflammation.
Oh Jueun, the first author of the study, explained, "This research is the first to experimentally clarify how AIM2 triggers the inflammatory response during Mpox virus infection."
The study also newly revealed that IRF1 is a protein (transcription factor) that regulates the amount of AIM2 within cells. IRF1 initiates the synthesis of AIM2 protein by binding to the DNA region involved in AIM2 production.
The research team also verified whether inhibiting AIM2 could be effective in treating severe inflammatory responses. When an AIM2 inhibitor was administered to mice, inflammation and cell death in lung tissue were alleviated, and the survival rate increased compared to the group that did not receive the inhibitor.
Professor Lee Sangjun stated, "These results suggest that AIM2 could serve as a new therapeutic target for alleviating severe inflammatory pathological responses in emerging infectious diseases." He added, "However, since AIM2 also plays a role in alerting the immune system to external invaders, excessive inhibition could reduce the body's ability to eliminate viruses, so therapeutic approaches must take this into account."
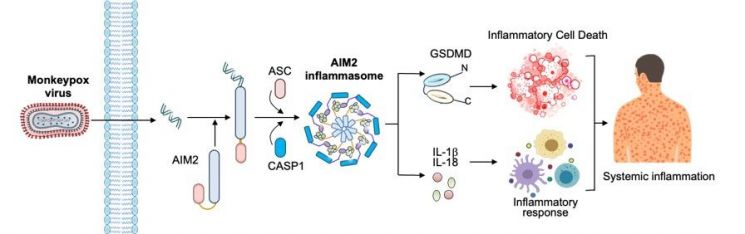 The process by which the AIM2 protein sensor triggers an inflammatory response upon infection with the Mpox virus.
The process by which the AIM2 protein sensor triggers an inflammatory response upon infection with the Mpox virus.
The research findings were published on November 12 in 'Cellular & Molecular Immunology,' a leading journal in the field of immunology.
The research was supported by the Korea Drug Development Fund (KDDF), the National Research Foundation of Korea (NRF), the Korea Health Industry Development Institute (KHIDI), the Institute for Basic Science (IBS), the Donggrami Foundation, research funds from Ulsan National Institute of Science and Technology (UNIST), the Korean Society of Ginseng, Yuhan Corporation, the Korea National Institute of Health, and the Center for Women in Science, Engineering and Technology (WISET).
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














