UNIST, KRICT, and KETI Achieve Fundamental Suppression of Reactive Oxygen Species Generation
Development of Polymer-Based Semi-Solid Electrolyte
Gas Suppression Reduces Battery Swelling to One-Sixth
Published in Adv. Energy Mater.
A gel-type material has been developed that extends the lifespan of 'high-voltage batteries' for long-range electric vehicles while reducing the risk of explosion.
This material completely blocks the generation of reactive oxygen species, which are the main cause of 'aging' in high-voltage batteries. When applied, battery lifespan increased by 2.8 times, and swelling was reduced to about one-sixth of the original level.
The research team led by Professor Song Hyungon from the Department of Energy and Chemical Engineering at UNIST, in collaboration with Dr. Jung Seohyun of the Korea Research Institute of Chemical Technology and Dr. Hwang Chihyun of the Korea Electrotechnology Research Institute, announced on November 4 that they have developed an 'anthracene-based semi-solid gel electrolyte (An-PVA-CN)' that fundamentally blocks the reaction in which reactive oxygen species leak from the electrode during high-voltage charging.
 Research team (from left) Professor Hyungon Song of UNIST, Dr. Seohyun Jung of Korea Research Institute of Chemical Technology, Dr. Chihyun Hwang of Korea Electrotechnology Research Institute, Researcher Jeongin Lee of UNIST (first author). Provided by UNIST
Research team (from left) Professor Hyungon Song of UNIST, Dr. Seohyun Jung of Korea Research Institute of Chemical Technology, Dr. Chihyun Hwang of Korea Electrotechnology Research Institute, Researcher Jeongin Lee of UNIST (first author). Provided by UNIST
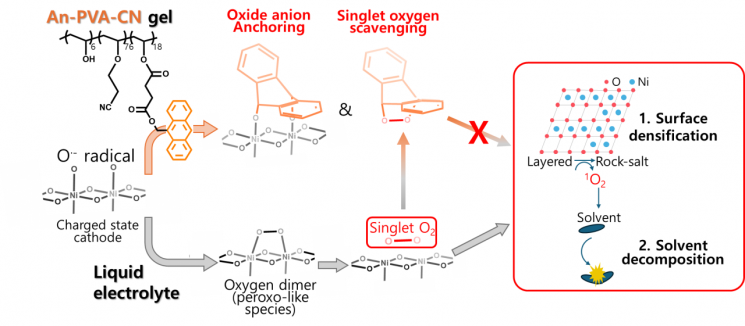
High-voltage batteries are lithium-ion batteries charged at voltages above 4.4V, allowing them to store more electricity and thus reduce battery pack weight. However, as charging voltage increases, the oxygen in the high-nickel cathode becomes unstable and transforms into a reactive oxygen species called 'singlet oxygen,' which escapes from the electrode. This reactive oxygen species generates gas, increasing the risk of battery explosion and shortening battery lifespan.
The anthracene (An) in the developed electrolyte binds with unstable oxygen on the electrode surface, blocking the reaction step where unstable oxygen atoms bond with each other. If unstable oxygen atoms combine, they form oxygen dimers, which are the 'seeds' of reactive oxygen species. Additionally, anthracene captures and removes already-formed reactive oxygen species, providing a dual protective function.
Another component of the electrolyte, the nitrile (-CN) functional group, stabilizes the nickel metal in the cathode, preventing nickel dissolution and structural deformation of the cathode.
Jeongin Lee, the first author of the study, explained, "The key differentiator of this research is that it blocks the generation stage of reactive oxygen species itself. Previously, approaches focused on neutralizing reactive oxygen species after they had already formed using antioxidants, or on manipulating the electrode to suppress oxygen generation."
Batteries using the new electrolyte maintained 81% of their initial capacity even after 500 charge-discharge cycles under a high-voltage condition of 4.55V, whereas conventional batteries dropped below 80% of their initial capacity after just 180 cycles. Since a battery is considered to have reached the end of its life when its capacity falls below 80% of the initial value, this means the lifespan increased by 2.8 times.
Gas generation, which causes battery swelling, was also significantly suppressed. While conventional batteries expanded by 85 micrometers (μm), batteries with the gel electrolyte swelled by only about 13 μm, reducing volumetric expansion to approximately one-sixth of the original level.
Professor Song Hyungon stated, "This research demonstrates that the oxygen reactions in high-voltage batteries can be directly controlled at the 'electrolyte design' stage. This principle could be applied in the future to the development of lightweight lithium-ion batteries for aerospace and large-scale energy storage systems (ESS)."
This research was published online on October 5 in 'Advanced Energy Materials,' an international journal in the field of energy materials.
The study was supported by the InnoCore program of Hydro*Studio at UNIST, the Korea Institute for Advancement of Technology, and projects of the Korea Research Institute of Chemical Technology.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














