A gene-editing tool that can turn genes on and off like a light switch has been developed. Turning a gene on means activating the production of proteins or substances, while turning it off means suppressing their production. Until now, research in South Korea has mainly focused on the function of turning genes off. In contrast, this new study has developed a technology that allows genes to be freely switched on and off, presenting a new paradigm for the synthetic biology-based bioindustry.
KAIST announced on September 21 that a joint research team led by Professor Lee Jooyoung of the Graduate School of Bioengineering (also Department of Life Science) and Dr. Noh Myunghyun of the Korea Research Institute of Chemical Technology has developed a "dual-mode CRISPR gene-editing system" that can simultaneously turn specific genes on and off in Escherichia coli.
 (From left) Dr. Moon Sooyoung, KAIST Institute for Life Science, Professor Lee Jooyoung, Graduate School of Bioengineering (also Department of Life Science), Dr. Noh Myunghyun, Korea Research Institute of Chemical Technology, and Researcher Ann Nanyeong, Department of Life Science. Provided by KAIST
(From left) Dr. Moon Sooyoung, KAIST Institute for Life Science, Professor Lee Jooyoung, Graduate School of Bioengineering (also Department of Life Science), Dr. Noh Myunghyun, Korea Research Institute of Chemical Technology, and Researcher Ann Nanyeong, Department of Life Science. Provided by KAIST
Gene-editing technology is regarded as one of the most innovative tools in 21st-century biotechnology. However, existing gene-editing tools have mainly specialized in the "off" (suppression) function, making them effective at blocking gene expression but limited in their ability to turn genes on.
Furthermore, for gene-editing tools to function, a specific DNA recognition sequence called a protospacer adjacent motif (PAM) is required. Existing systems have a narrow range of PAM recognition, which limits the number of genes that can be regulated.
The core of synthetic biology lies in designing the genetic circuits of living organisms as if programming them to perform desired functions.
In this process, bacteria, which serve as the foundation of synthetic biology, have simple structures and rapid proliferation rates, enabling the production of various useful substances. Utilizing these characteristics, activating genes within bacteria is considered a key technology for designing "microbial factories," and its industrial value is highly regarded.
However, while gene-editing-based activation (turning on) has made progress in eukaryotic cells such as those of humans, plants, and animals, it has not functioned properly in bacteria due to differences in internal transcriptional regulation mechanisms.
This is precisely why technology that can optimize metabolic pathways by activating certain genes and suppressing others-like turning lights on and off with a switch-has been needed.
The dual-mode gene-editing tool developed by the joint research team has overcome previous limitations, becoming a core tool for precise genetic regulation. The system advances existing gene-editing tools, which were focused on turning genes off, to enable both activation and suppression simultaneously.
In particular, when the performance of the dual-mode gene-editing tool was tested, gene expression increased by 4.9 times when genes were turned on, and expression was suppressed by up to 83% when turned off. The ability to simultaneously regulate two different genes (turning one on and the other off) is cited as the greatest advantage of the gene-editing tool developed by the joint research team.
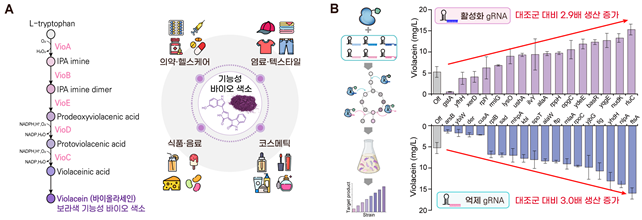 Biosynthetic Pathways of Violacein and Expected Production Increase Effects in Industrial Applications. Provided by KAIST
Biosynthetic Pathways of Violacein and Expected Production Increase Effects in Industrial Applications. Provided by KAIST
To demonstrate the practical utility of the dual-mode gene-editing tool, the joint research team also attempted to increase the production of violacein, a purple pigment with anticancer effects. As a result, in large-scale experiments conducted on all genes in Escherichia coli, the dual-mode gene-editing tool enabled the identification of genes that are effective for violacein production.
For example, the experiment confirmed that turning on the "rluC" gene, which aids in protein production, increased output by 2.9 times, while turning off the "ftsA" gene, which is involved in cell division, increased output by 3.0 times. Furthermore, when both genes were regulated simultaneously (one turned on and the other off), a greater synergistic effect was observed, with production increasing by approximately 3.7 times, according to the research team.
Professor Lee stated, "This research is significant in that it combines gene-editing and synthetic biology to enhance the efficiency of microbial production platforms," adding, "The achievement of controlling complex gene networks with a single system represents a new research paradigm."
He further noted, "The technology developed by the joint research team has also been confirmed to work in other bacterial species, making it applicable in various fields such as biopharmaceuticals, chemicals, and fuel production."
Meanwhile, this study was conducted with Dr. Moon Sooyoung, a postdoctoral researcher at the KAIST Institute for Life Science, as the first author. The research results were recently published online in the molecular biology journal "Nucleic Acids Research."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














