Strengthening Leadership as a Biosimilar Pioneer Through Early Market Entry
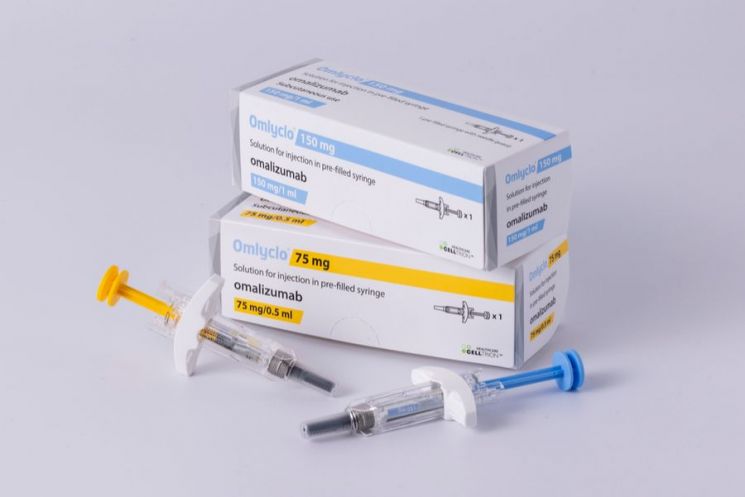
Celltrion announced on September 19 that it has launched Omriclo (ingredient: omalizumab), a treatment for chronic spontaneous urticaria, in Europe.
Omriclo is the first omalizumab biosimilar product to be launched in Europe. Celltrion plans to leverage its status as a "first mover" to actively utilize its direct sales capabilities in the region and quickly capture market share. The term "first mover" refers to a company that is the first to enter a new market or technology sector.
Celltrion first launched Omriclo in Norway, a key country in Northern Europe. In Norway, omalizumab treatments are typically supplied through retail channels. Taking into account these market characteristics, Celltrion's local subsidiary has been accelerating Omriclo sales by expanding communication with major channels such as pharmacies following the product launch.
Starting with Norway, the launch of Omriclo is expected to expand to the five major European countries (EU5) and neighboring countries from the fourth quarter of this year. In particular, Celltrion is proactively pursuing launches in major markets such as Germany, France, and Spain. As competing biosimilar products are expected to enter the market later, Omriclo's advantage as a first mover is expected to become even more pronounced.
In Europe, many countries use a bidding system that grants exclusive supply rights, making the competitiveness of first mover products stand out more clearly than in other regions. If a company launches its product before competitors, it can participate in exclusive bids and secure the market for a certain period. The prescription data and preferences from healthcare professionals and patients gained during this period can serve as favorable indicators in future tenders, further accelerating market share growth.
Ha Taehoon, Head of Celltrion Europe, stated, "We are actively leveraging Omriclo's first mover advantage to communicate smoothly with bidding agencies in each country. Starting with Norway, we plan to quickly launch the product in major European countries to secure early market leadership."
He added, "Celltrion has established a unique brand identity in the fields of autoimmune and oncology treatments in Europe. With Omriclo, we will also provide high-quality biopharmaceuticals for dermatological diseases at reasonable prices, further building trust with healthcare professionals and patients."
Meanwhile, Omriclo, developed by Celltrion, is used to treat chronic spontaneous urticaria and asthma. The original drug, Xolair, recorded global sales of approximately 6.4992 trillion won last year.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














