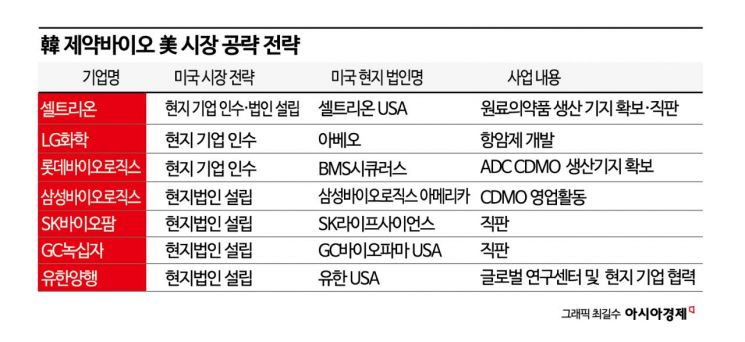
Celltrion Pursues Acquisition of cGMP Production Facility
Samsung Biologics and Others Also Considering U.S. Investments
Trump-Driven Tariff War
Changing the Export Formula for Korean Manufacturing and Local Demand
The "tariff war" triggered by U.S. President Donald Trump is fundamentally transforming the production and supply mechanisms of South Korea's pharmaceutical and biotech industries. A clear symbol of this shift is Celltrion, a leading Korean biotech company, which is moving quickly to acquire a biopharmaceutical plant in the United States for a massive sum of 700 billion won. At this point, it is difficult to precisely measure how the Trump administration’s soon-to-be-announced pharmaceutical tariff rates will affect individual companies. However, given the increased volatility of the world’s largest market?the United States?there are growing expectations that the industry’s push for "localization" will accelerate, as companies seek to absorb the unpredictable shocks and turmoil of the global market that may arise at any time.
According to industry sources on August 6, President Trump stated in an interview with CNBC the previous day (local time) that "pharmaceutical tariffs will be raised up to 250%." He specifically explained, "Initially, a 'small tariff' will be imposed on imported pharmaceuticals, and within a year and a half, it will rise to 150%, and then to 250%," adding, "because we want medicines made in our own country (the United States)." The exact rate of the initial "small tariff" mentioned by Trump was not disclosed. Previously, the governments of Korea and the United States had promised "most-favored-nation" treatment for pharmaceutical tariffs during mutual tariff negotiations. As a result, Korean pharmaceuticals are not expected to be subject to the highest tariff bracket, but the explicit demand for local production in the U.S. through the announcement of high tariffs has led the pharmaceutical and biotech industry to react by saying, "The inevitable has come."
Until now, Korean pharmaceutical and biotech companies have maintained a strategy of mass-producing active pharmaceutical ingredients (APIs) domestically and exporting them to advanced markets such as the United States and Europe, while only final packaging and distribution were handled locally. This was possible due to Korea's competitive production costs, robust Good Manufacturing Practice (GMP) infrastructure, and a skilled workforce. Production bases were concentrated in bio clusters such as Songdo in Incheon and Osong in North Chungcheong Province to maximize efficiency, with products rapidly transported by air to meet demand.
However, amid the Trump administration’s anti-free trade or protectionist policies, the need to localize a significant portion of production has grown considerably. Celltrion is the company most proactively and swiftly pursuing this change. Celltrion has been selected as the preferred bidder, ahead of two global companies, in the bid to acquire a biopharmaceutical manufacturing plant in the United States, and is now on the verge of securing a production base in the country. This facility has produced major biopharmaceuticals such as anticancer drugs and treatments for autoimmune diseases for several years.
Celltrion’s decision carries significance beyond a simple investment. It could signal a shift for the Korean pharmaceutical and biotech industry from its traditional "domestic-based export model" to a "global production and localization" model, as it aims to become a "global company." Leading global big pharma and contract development and manufacturing organizations (CDMOs) operate multiple production bases and employ customized manufacturing strategies to respond to local regulations and demand. Among Korean companies, Samsung Biologics and SK Pharmteco are also considering investments in the United States.
Celltrion initially considered building a new plant, but after assessing the high construction costs, shortage of skilled labor, and weak manufacturing infrastructure locally, the company decided to acquire an existing manufacturing company instead. Celltrion is also considering further expansion after acquiring a local production base.
The advantages of securing a local plant are clear. The entry barriers to the U.S. biopharmaceutical market are lowered. Although the U.S. Food and Drug Administration (FDA) has a complex and stringent approval process, having local manufacturing infrastructure accelerates the overall commercialization process?from approval to distribution, insurance coverage, and hospital adoption. Logistics costs can also be saved. In addition, if the U.S. federal or local governments provide tax benefits, subsidies, or research and development (R&D) support to companies investing in biomanufacturing facilities, there could be substantial advantages that are difficult to quantify.
However, some concerns have been raised within the industry. The biggest issue is the potential reduction of Korea’s domestic biomanufacturing base. Until now, large-scale bio-industrial complexes have been established in areas such as Songdo, Ochang, and Osong, but if companies relocate core production facilities to the United States, the economic effects?such as employment?generated by clusters like Songdo and Ochang could quickly diminish. Moreover, production costs in the United States, including labor, construction, and maintenance, are much higher than in Korea, which could weigh on corporate profitability. Especially in biopharmaceuticals, the initial investment in production lines and workforce training is enormous, making it difficult to achieve a quick turnaround in profits.
Lee Seungkyu, Vice Chairman of the Korea Bio Association, said, "Operating a plant in the United States may not be economically viable due to construction costs and high labor expenses," adding, "Even if investment proceeds, it will be necessary to negotiate incentives to ensure economic feasibility."
Despite these drawbacks associated with localization, the general view is that Korean companies’ efforts to diversify their production and supply systems will only accelerate. If the Trump administration’s persistent tariff and non-tariff pressures?having designated pharmaceuticals as a core advanced manufacturing technology?continue for some time, a restructuring of the business ecosystem to adapt will be inevitable. Once reorganized, it will be difficult to revert the production and supply system to its previous state. If direct local production of active pharmaceutical ingredients (DS) proves difficult, companies are likely to respond by increasing the proportion of contract manufacturing for DS and finished pharmaceuticals (DP).
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.