ImmuneOncia's Stock Price Declines After May 19 Listing
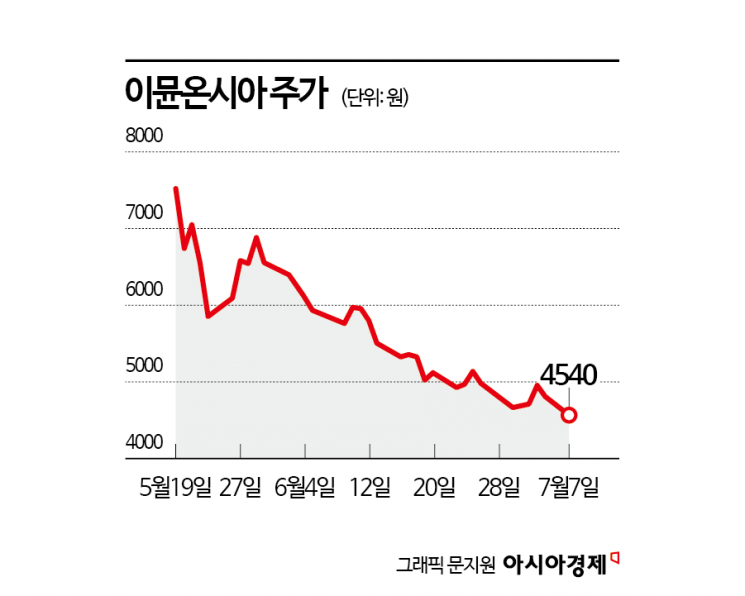
Shares Drop from 8,300 Won on Debut to 4,540 Won
Stock Price Expected to Rebound Only If Hopes for Technology Transfer Revive
The majority of newly listed companies entering the KOSDAQ market initially attract attention but are soon neglected by institutional investors. The stock price of ImmuneOncia, which drew interest even before its listing as a subsidiary of Yuhan Corporation, also surged on the day of its debut but has since declined for several consecutive days.
According to the financial investment industry on July 8, ImmuneOncia's stock price has fallen by 45.3% compared to its all-time high of 8,300 won recorded on its listing day, May 19.
Since ImmuneOncia’s listing, domestic institutional investors and foreign investors have recorded cumulative net sales of 64 billion won and 12.2 billion won, respectively. Individual investors have purchased shares worth 91 billion won. The average purchase price for individual investors was 6,870 won, resulting in an average unrealized loss rate of -34%.
Founded in 2016, ImmuneOncia is developing immune checkpoint inhibitors targeting T cells and macrophages. Its largest shareholder is Yuhan Corporation, which holds a 66.97% stake.
The company’s main pipeline includes the programed cell death protein-ligand 1 (PD-L1) monoclonal antibody ‘IMC-001’ and the next-generation cluster of differentiation 47 (CD47) monoclonal antibody ‘IMC-002’. Its core assets are anti-PD-L1, anti-CD47, and anti-LAG-3 monoclonal antibodies. ImmuneOncia has established a business model that generates revenue by developing antibody-based drug candidates and out-licensing them at the early clinical stage.
IMC-001 is an antibody therapy that works by inhibiting the immune checkpoint protein PD-L1. It is intended for the treatment of NK-T cell lymphoma, a rare blood cancer, and the company plans to apply for orphan drug designation (ODD) from the Ministry of Food and Drug Safety in the second half of this year. If granted ODD status, the drug can be approved based solely on Phase 2 clinical results. IMC-001 is currently in Phase 2 clinical trials. In Phase 2 trials for NK/T cell lymphoma, IMC-001 achieved an objective response rate of 79%. IMC-002 has demonstrated safety in a Phase 1a trial for solid tumors.
At the time of listing, ImmuneOncia projected that it would begin generating significant revenue starting next year. ImmuneOncia CEO Kim Heungtae promised at the time of the IPO, “Starting with global technology transfer in 2026, we will become a company that creates meaningful momentum every year.”
For IMC-002, the company expects to enter Phase 1b clinical trials in China next year. Under the technology transfer agreement with 3D Medicines, ImmuneOncia can receive a milestone payment of 5.3 billion won next year. The company also anticipates the possibility of a global technology transfer of IMC-002 next year, with the contract size estimated at 17.1 billion won.
The financial investment industry expects that concrete developments related to technology transfer will serve as a catalyst for a rebound in the stock price.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














