Professor Junhyuk Seo's Chemistry Research Team
Announces Breakthrough in Hydrogen Evolution Reaction
with Highly Oxidized Metal Catalysts
Research findings that mark a significant turning point in the development of next-generation electrochemical catalysts for hydrogen production have been announced. A Korean research team has identified a new reaction principle and pathway for the hydrogen evolution reaction (HER) that occurs when metals are in a highly oxidized state, where they have lost many electrons, drawing notable attention.
Gwangju Institute of Science and Technology (GIST) announced on the 16th that a research team led by Professor Junhyuk Seo of the Department of Chemistry has demonstrated how 'hydrogen bonding' can facilitate the hydrogen evolution reaction, using a tungsten (W) metal complex with a unique ligand molecule called dithiolene.
A highly oxidized state refers to a condition where a metal atom possesses a higher oxidation state than usual, meaning it has lost more electrons. Metals in such a state exhibit strong electron-withdrawing properties and can display unique reactivity or catalytic activity in electrochemical reactions. In electrocatalytic reactions such as the hydrogen evolution reaction, a highly oxidized state can play a crucial role by inducing new reaction pathways or enhancing reaction efficiency.
This study elucidated the working principle of the hydrogen evolution reaction using highly oxidized metals, providing a new direction for designing next-generation catalysts. In particular, it confirmed that the interaction between the metal and the ligands bound around it is extremely important when the catalyst is active, and demonstrated that surrounding molecules, which had not previously received much attention, can have a decisive impact on actual reaction efficiency.
The hydrogen evolution reaction is a core technology for utilizing hydrogen gas as an eco-friendly energy source. While previous research mainly focused on the electronic structure of the metal at the center of the catalyst, recent attention has shifted toward the role of molecules attached around the metal, which can modify the metal's properties and regulate the reaction.
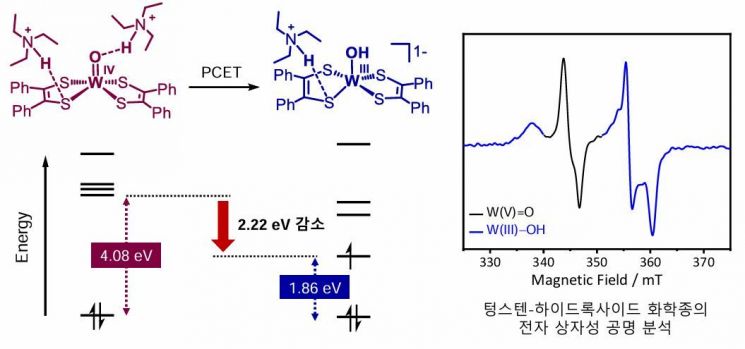 Molecular orbital energy level reduction phenomenon caused by hydrogen bonding (left) and tungsten-hydroxide chemical species first observed through electron paramagnetic resonance (EPR) analysis.
Molecular orbital energy level reduction phenomenon caused by hydrogen bonding (left) and tungsten-hydroxide chemical species first observed through electron paramagnetic resonance (EPR) analysis.
Dithiolene is a ligand well known for stabilizing metal ions, but it had not been experimentally confirmed that this molecule could bond with hydrogen and assist in proton transfer in highly oxidized tungsten compounds.
The research team experimentally demonstrated for the first time in the world that, within the tungsten complex, a weakly acidic substance forms hydrogen bonds simultaneously with the oxygen (W=O) atom bound to the metal and the sulfur (S) atom of the dithiolene molecule. This dual hydrogen bonding enables the simultaneous transfer of electrons and protons, creating an electronic structure that facilitates the hydrogen evolution reaction.
Through single-crystal X-ray structural analysis, the team confirmed that when the weakly acidic substance triethylammonium was added to the compound, hydrogen bonds were simultaneously formed with both the oxygen of the metal and the sulfur atom of the dithiolene molecule.
During this process, the electronic structure of the molecule changed, allowing electrons to move more easily. As a result, the reduction reaction of tungsten (W(IV) to W(III)) became possible at a lower voltage than before, effectively reducing the energy required for the hydrogen evolution reaction.
Additionally, using electron paramagnetic resonance (EPR) analysis, the team succeeded in directly detecting the W(III)?OH intermediate formed after hydrogen bonding. This provides decisive evidence that the 'proton-coupled electron transfer (PCET)' mechanism, in which electrons and protons move together, actually occurred.
Experiments using deuterium (D), a heavier isotope of hydrogen (H), also showed a difference in reaction rate (H/D ratio of 1.62), proving that the proton transfer process via hydrogen bonding directly affects the reaction rate.
Theoretical calculations (DFT) also demonstrated that dual hydrogen bonding effectively stabilizes the electronic structure of the molecule, allowing it to act as the active species in actual catalytic reactions.
The catalytic performance was also verified experimentally: the tungsten complex achieved a Faradaic efficiency of up to 99% and a turnover frequency (TOF) of approximately 122,277 per second, demonstrating excellent hydrogen production capability.
Professor Junhyuk Seo stated, "Through this research, we have experimentally proven that molecules bound around the metal not only stabilize the metal but also play a practical role in facilitating the movement of electrons and protons. This enables a deeper understanding and explanation of the fundamental principles of next-generation energy conversion reactions such as artificial photosynthesis, carbon dioxide conversion, and water electrolysis technologies."
This research was supervised by Professor Junhyuk Seo of GIST Department of Chemistry, Professor Jin Kim (Sunchon National University), Dr. Sunhee Kim (KBSI and Chung-Ang University), and Professor Kyungbin Cho (Chonbuk National University). Wonjoong Lee, a doctoral student in the GIST Department of Chemistry, participated as the first author. The study was supported by the Ministry of Science and ICT, the National Research Foundation of Korea (NRF), the Ministry of Education's LAMP program, and the Korea Basic Science Institute (KBSI).
The results were published online on May 22 in the international journal 'Angewandte Chemie International Edition (ACIE)', issued by the German Chemical Society.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.