Aptozma, a Biosimilar of Actemra,
Launched as a "First Mover" in the Domestic Market
On June 2, Celltrion Pharm announced that it has launched domestic sales of Aptozma, a biosimilar of Actemra (active ingredient: tocilizumab), a treatment for autoimmune diseases.
Aptozma is an interleukin inhibitor that reduces inflammation by suppressing the interleukin (IL)-6 protein, which is involved in triggering inflammation in the body. It is approved for indications including rheumatoid arthritis (RA), systemic juvenile idiopathic arthritis (sJIA), and polyarticular juvenile idiopathic arthritis (pJIA). The domestic market size is estimated to be approximately 20 billion KRW.
According to the global Phase 3 clinical trial of Aptozma (project name CT-P47) conducted by its developer, Celltrion, on a total of 471 patients with rheumatoid arthritis, Aptozma met the predefined equivalence criteria compared to the original drug, confirming its efficacy, equivalence, pharmacokinetics, and safety.
Based on these clinical results, Celltrion obtained product approvals for Aptozma in the United States and Europe in January and February, respectively. In December of the previous year, the intravenous (IV) formulation "Aptozma IV" was also approved in Korea as the first tocilizumab biosimilar, securing a "First Mover" status in the domestic market.
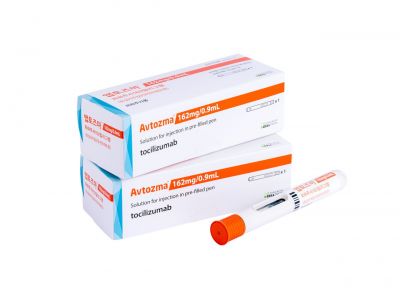
Furthermore, in February of this year, the subcutaneous (SC) formulation "Aptozma Subcutaneous Injection (162mg)" received product approval. After being listed at a price of 245,938 KRW, Celltrion Pharm is launching it in Korea this month, which will allow the company to gain a competitive edge over rival biosimilars in the market.
Notably, the newly launched Aptozma Subcutaneous Injection (Autoinjector type) features an improved syringe design that enhances convenience and safety for administration. Unlike competing products that require pressing an external button to inject the drug, Aptozma Subcutaneous Injection allows for immediate administration simply by pressing the syringe against the skin. This resolves concerns about drug loss due to issues such as incorrect injection angles during administration. In addition, after a separate stability test, the shelf life has been extended to 36 months from the date of manufacture, which is about 12 months longer than the 24-month shelf life of competing products.
Celltrion Pharm plans to secure a competitive advantage through the rapid launch of this SC formulation and is committed to improving patient access to treatment. The company also plans to hold briefing sessions for healthcare professionals to expand its market share. Furthermore, by the end of this year, Celltrion Pharm intends to launch the intravenous (IV) formulation of Aptozma (80mg, 200mg, 400mg) so that healthcare professionals can selectively prescribe according to the patient's condition and convenience.
Including this product, the number of biosimilar products sold by Celltrion Pharm in Korea has increased from six (Remsima, Remsima SC, Truxima, Herzuma, Yuflyma, Begzelma) at the time of the Vision 2030 declaration in August of last year to a total of twelve, with six new products (Stoboclo, Ocenbelt, Idengelt, Stekima, Omriclo, Aptozma) recently added. With the product portfolio doubling in just one year, significant sales growth in the biosimilar sector is expected in the future.
A Celltrion Pharm representative stated, "Aptozma will quickly establish itself in the domestic market by leveraging its enhanced convenience and First Mover advantage," adding, "We expect significant growth in the biosimilar sector as we greatly expand our product portfolio this year."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














