(From left) Professor Hyunseop Lim, Department of Chemistry, GIST; Professor Seokwon Hong, Department of Chemistry, GIST; Professor Jongwook Hong, KENTECH; PhD candidate Hyunju Kim, Department of Chemistry, GIST.
(From left) Professor Hyunseop Lim, Department of Chemistry, GIST; Professor Seokwon Hong, Department of Chemistry, GIST; Professor Jongwook Hong, KENTECH; PhD candidate Hyunju Kim, Department of Chemistry, GIST.
On May 20, the Gwangju Institute of Science and Technology (GIST) announced that a joint research team led by Professor Hyunseop Lim from the Department of Chemistry at GIST and Professor Jongwook Hong from the Korea Institute of Energy Technology (KENTECH) has developed a next-generation fuel cell catalyst with superior electrochemical performance and durability compared to conventional commercial platinum catalysts (Pt/C), utilizing a palladium selenide-based mixed-phase nanostructure for the oxygen reduction reaction (ORR).
This research focused on the synergistic effects that arise when different crystal phases coexist in a mixed-phase material. The study is significant because it presents a new direction for designing high-performance and highly durable catalysts that could replace conventional platinum-based electrocatalysts. The findings are expected to make an important contribution to the effective design strategies of mixed-phase materials for advanced energy conversion technologies such as fuel cells in the future.
Palladium selenide (Pd-Se) is a compound formed by the combination of palladium (Pd) and selenium (Se), and its various crystal structures provide properties suitable for electrochemical reactions. In particular, it exhibits high catalytic activity for the oxygen reduction reaction in fuel cells, making it a promising next-generation catalyst to replace platinum (Pt). The mixed-phase structure, where multiple crystal phases coexist, enhances both performance and durability through synergistic effects.
Until now, most research on Pd-Se catalysts has focused on single crystal phases, and it was largely unknown that interactions between different Pd-Se crystal phases could contribute to improved catalytic performance.
In response, the research team newly revealed that the mixed-phase structure facilitates electron transfer pathways and effectively forms active sites for reactions.
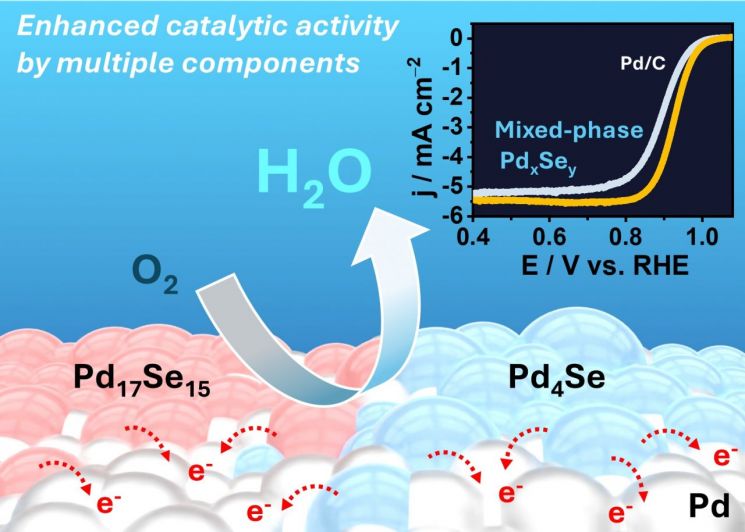
The core of the research lies in designing a single organometallic precursor and precisely heat-treating it, without complex processes, to synthesize a 'mixed-phase nanostructure' in which various Pd-Se crystal phases (such as PdSe2, Pd17Se15, and Pd4Se) coexist. The synergistic effects arising from electronic structure interactions at the interfaces between different crystal phases resulted in an increased reaction rate for the oxygen reduction reaction and reduced energy loss. As a result, the mixed-phase material with multiple coexisting crystal phases demonstrated superior reaction efficiency and durability compared to conventional single-phase Pd/C or Pt/C catalysts.
It is noteworthy that the research team proved through theoretical calculations and experiments that different Pd-Se crystal phases exhibit their respective strengths at different stages of the oxygen reduction reaction, thereby increasing overall reaction efficiency and minimizing energy loss.
The Pd-Se catalyst synthesized at 1,000°C achieved a half-wave potential of 0.931V for the oxygen reduction reaction, which is higher than that of the commercial platinum-based catalyst (Pt/C). This indicates that oxygen can be reduced with less energy under the same conditions, serving as a representative indicator of the excellent electrochemical activity of the Pd-Se catalyst.
The research team used Density Functional Theory (DFT) to calculate the electronic structures of each Pd-Se crystal phase and discovered that each phase optimizes different intermediate steps in the oxygen reduction reaction process, thereby enhancing catalytic performance.
Through this, the team confirmed that the three Pd-Se materials play complementary roles in the oxygen reduction reaction (ORR) process. Specifically, Pd is responsible for the initial adsorption of oxygen molecules; Pd17Se15 stabilizes intermediates in the early stages of the reaction to prevent interruption; and Pd4Se stably adsorbs intermediates in the subsequent stages, thereby increasing the overall reaction rate.
Professor Hyunseop Lim stated, "This study is an example that experimentally and theoretically demonstrates that different Pd-Se crystal phases can harmonize to optimize each stage of the electrochemical reaction," adding, "This technology has great potential to be utilized as a core technology in next-generation eco-friendly energy fields such as high-performance fuel cells, metal-air batteries, and water electrolysis systems."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.