After Biotech Firms, Hanmi Pharmaceutical Joins Collaboration
Strategy for Patent Extension and Indication Expansion
Keytruda, the immune-oncology drug from Merck (MSD), which holds the number one spot in global sales, is rapidly expanding its collaborations with South Korean pharmaceutical and biotech companies. While previous combination clinical trials with Keytruda (simultaneous administration of two drugs) were mostly conducted with biotech firms specializing in new drug development, Hanmi Pharmaceutical has now joined these efforts.
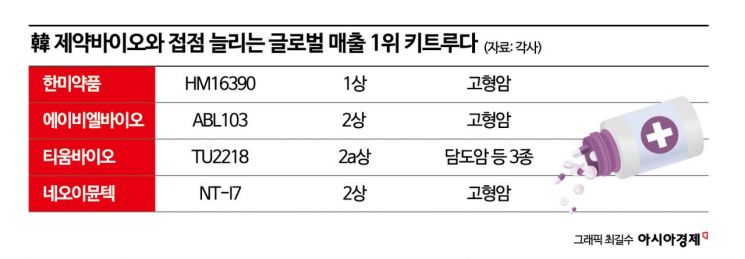
According to industry sources on May 20, Hanmi Pharmaceutical recently initiated a Phase 1 clinical trial combining its next-generation IL-2-based immuno-oncology drug, HM16390, with Keytruda. Hanmi Pharmaceutical is acting as the sponsor and is overseeing the trial, while MSD is supplying Keytruda free of charge for the study. According to MSD Korea, including Hanmi Pharmaceutical, there have been 14 domestic joint clinical collaborations related to Keytruda combination therapies with 11 companies since 2020.
Hanmi Pharmaceutical’s new drug, HM16390, is designed based on the cytokine interleukin-2 (IL-2), which regulates the differentiation and proliferation of immune cells. It is a treatment that helps the body’s immune cells grow effectively and fight cancer cells. While the existing IL-2 drug, Proleukin, was associated with severe side effects due to high toxicity, HM16390 has been developed as a variant to reduce toxicity and enhance efficacy. This drug increases T cells, which are immune cells within the tumor microenvironment (TME). In tumor microenvironments where immune cells are unable to penetrate or respond effectively?a state known as a "cold tumor"?HM16390 helps convert this to a "hot tumor" state, thereby inducing an immune response. A hot tumor refers to a state in which immune cells have infiltrated the tumor microenvironment and are actively attacking cancer cells, in contrast to a cold tumor.
In addition to Hanmi Pharmaceutical, MSD is conducting various combination clinical trials with promising domestic biotech companies through partnerships. ABL Bio is conducting a Phase 1b/2 trial combining its bispecific antibody ABL103, which targets both 4-1BB and B7-H4 for solid tumor treatment, with Keytruda. TiumBio is conducting a Phase 2a combination trial with Keytruda to secure an indication for biliary tract cancer. NeoImmuneTech’s immune cell enhancer NT-I7 is also in a Phase 2 combination trial with Keytruda. These companies are conducting joint clinical trials with Keytruda supplied free of charge by MSD. The leadership in the immuno-oncology market is rapidly shifting from monotherapies to combination therapies. The expanding collaboration between MSD and domestic companies demonstrates the growing stature of the Korean bio industry on the global stage. It also serves as a strategic touchpoint for responding to market changes following Keytruda. Jung Yoon-taek, head of the Pharmaceutical Industry Strategy Research Institute, stated, "The fact that MSD is conducting joint clinical trials means that these companies have been selected by MSD. For domestic pharmaceutical companies, the collaboration itself serves as an indirect promotion of their capabilities to the global market."
From MSD's perspective, this is also a move to mitigate risks following patent expiration. Major countries are scheduled to see Keytruda’s patents expire between 2028 and 2030, making it urgent to secure new combination patents through combination therapies. This strategy aims to maintain market share even after patent expiration. Since receiving FDA approval in 2015, Keytruda has secured indications for more than 20 types of cancer, including lung cancer, melanoma, renal cell carcinoma, and bladder cancer, making it the global sales leader. Last year, Keytruda generated $29.482 billion (approximately 41 trillion won) in sales, an 18% increase from 2023.
Another strategic advantage of combination therapies is their ability to target cancer types for which existing treatments have been less effective. While Keytruda alone induces a strong immune response, tumors possess various mechanisms to evade immune reactions, making combination strategies necessary to overcome these barriers. For example, combining Keytruda with drugs that suppress the tumor microenvironment or with biologics that activate immune cells can lower "immune resistance" and increase response rates. Lee Seung-kyu, Executive Vice Chairman of the Korea Biotechnology Industry Organization, stated, "The development of combination therapies could make it possible to prescribe treatments to patients who previously could not receive them. If such combination therapies are utilized, the original drug can retain its advantages even after its patent expires." Reported by Jung Donghun and Choi Taewon.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














