 (From left) Professor Joon Cho, Department of Biomedical Science and Engineering, GIST; Professor Mi Hye Lee, Soonchunhyang University Soonchunhyang Biomedical Research Institute; student Daehwa Yoon, GIST; student Boseon Kim, Soonchunhyang University; student Dahi Jung, GIST.
(From left) Professor Joon Cho, Department of Biomedical Science and Engineering, GIST; Professor Mi Hye Lee, Soonchunhyang University Soonchunhyang Biomedical Research Institute; student Daehwa Yoon, GIST; student Boseon Kim, Soonchunhyang University; student Dahi Jung, GIST.
A Korean research team has discovered a new fact: during the differentiation of adipocytes, gene translation and cellular metabolism interact closely and influence each other.
On April 24, the Gwangju Institute of Science and Technology (GIST) announced that a joint research team led by Professor Joon Cho of the Department of Biomedical Science and Engineering at GIST and Professor Mi Hye Lee of the Soonchunhyang University Soonchunhyang Biomedical Research Institute has, for the first time, identified two previously unknown mechanisms of reciprocal regulation between gene translation and metabolism. This was achieved through a multiomics analysis that integrates transcriptome, translatome, and proteome data.
Adipocytes are divided into white adipocytes, which store energy, and brown and beige adipocytes, which consume energy and generate heat. In particular, beige adipocytes originate from white adipocytes and, when stimulated by exercise or cold, acquire characteristics similar to brown adipocytes. They have been highlighted as promising targets for the treatment of metabolic diseases.
However, most previous research has focused primarily on the early stage in which genes are transcribed into RNA, while comprehensive analyses of the translation stage?where actual proteins are produced?or of the proteome itself have been insufficient.
To address this, the research team adopted a multiomics approach that integratively analyzes transcriptome, translatome, and proteome data obtained under identical conditions. This allowed them to systematically track how gene translation and cellular metabolism are organically regulated during adipocyte differentiation.
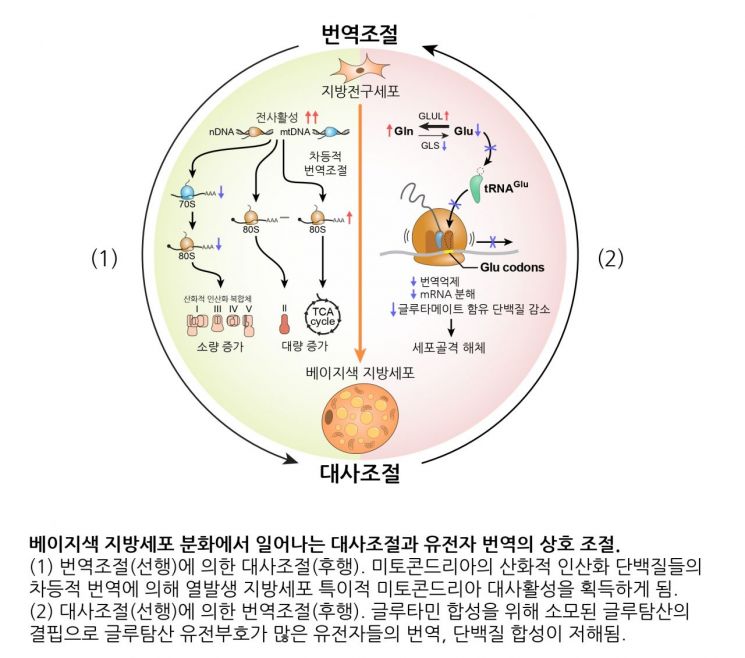 A novel mechanism of reciprocal regulation between protein translation and metabolism identified during beige adipocyte differentiation.
A novel mechanism of reciprocal regulation between protein translation and metabolism identified during beige adipocyte differentiation.
The researchers first analyzed the regulation of protein translation occurring in mitochondria, the key organelles responsible for energy production in cells. Their analysis revealed that, during adipocyte differentiation, the translation of genes encoding mitochondrial complexes I, III, IV, and V is suppressed, whereas complex II is excluded from this suppression and its relative proportion actually increases.
This result suggests that heat-generating beige adipocytes adjust the composition of their mitochondrial complexes to suit their metabolic characteristics, and that this regulation occurs precisely at the stage of protein synthesis.
The team also found that, during adipocyte differentiation, a decrease in glutamic acid caused by metabolic regulation leads to the suppression of the translation of proteins rich in this amino acid. They confirmed that, as the expression of genes involved in converting glutamic acid to glutamine increases during adipocyte differentiation, the concentration of glutamic acid decreases. As a result, ribosomes stall on mRNAs containing glutamic acid codons, suppressing protein production.
This ribosome stalling phenomenon inhibits the translation of genes with many glutamic acid codons, particularly reducing the production of proteins involved in cytoskeleton formation. Ultimately, this promotes the differentiation of adipocytes.
Professor Joon Cho of GIST stated, "This is the first experimental demonstration at the molecular level that metabolic substances involved in adipocyte differentiation can directly regulate gene translation. It shows that, within the complex biological process of adipocyte and tissue formation, metabolism and gene translation regulation are not independent and separate, but actively interact with each other."
Professor Mi Hye Lee of Soonchunhyang University said, "This study is significant in that it demonstrates that gene regulation associated with adipocyte metabolism is finely controlled not only at the transcriptional level but also at the translation stage. It is meaningful in revealing the importance of multilayered regulatory structures in adipocyte differentiation."
Meanwhile, these research findings were published online in the international journal Nature Communications on April 9.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














