National Biotech Initiative Act Introduced
Biosecurity Act Resurfaces
The United States is elevating biotechnology to a national strategic industry and intensifying pressure by unveiling regulatory measures to counter China. As a result, the restructuring of the global bio supply chain is expected to accelerate, and cautious voices are emerging that anticipate the Korean bio companies, equipped with world-class production capabilities, to benefit from this shift.
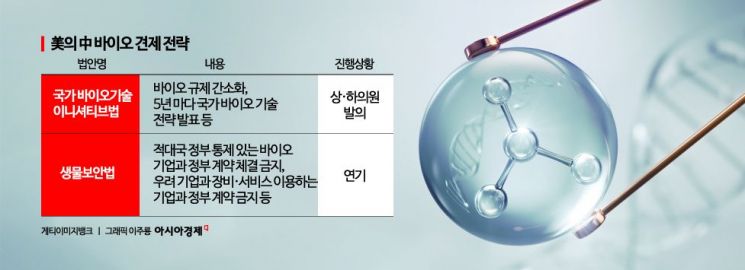
According to the Korea Bio Association on the 18th, the U.S. Congress recently introduced the "National Biotech Initiative Act" simultaneously in both the Senate and the House of Representatives. Key members from both the Republican and Democratic parties, including Senator Todd Young, participated in the bill's introduction. The bill aims to elevate the status of biotechnology as a national strategic industry and reduce technological dependence on strategic competitors, including China.
Specifically, the bill includes provisions to establish a strong control tower within the U.S. administration to promote and foster biotechnology. It mandates that the agency streamline biotechnology regulations and announce a national biotechnology strategy every five years. Additionally, it calls for the creation of an inter-agency committee to coordinate policies across federal departments and agencies, assigning clear roles and responsibilities related to biotechnology to all relevant federal entities.
The U.S. desire to strategically nurture the bio industry stems from considerable concerns. A report released earlier this month by the U.S. Senate Emerging Biotech National Security Committee warned that "China has prioritized biotechnology as a strategic focus for 20 years and is rapidly gaining an advantage in this field." China is a major exporter of active pharmaceutical ingredients and generic drugs to the U.S. Recently, global big pharma companies such as AstraZeneca and GlaxoSmithKline (GSK) have shown increased interest by acquiring rights to new drugs developed and clinically tested in China. Following trade wars and disputes over supply chains of critical resources like rare earth elements, tensions between the U.S. and China are expected to escalate sharply in the bio sector as well.
The "Biosecurity Act," which was postponed in the U.S. Congress last year, is also expected to be reconsidered. The Biosecurity Act aims to designate major companies such as China's Wuxi AppTec and Wuxi Biologics as "concerned bio companies" and prohibit all equipment and service contracts with U.S. companies in the future.
These moves by the U.S. government are likely to present positive opportunities for Korean bio companies, which hold a neutral position in the global bio supply chain. Particularly in the contract development and manufacturing organization (CDMO) sector, where China had strengths, companies like Samsung Biologics, Celltrion, and Lotte Biologics have already established high competitiveness through large-scale capacity expansions. An industry insider stated, "As the U.S. enters a full-fledged conflict with China in the bio sector, the trend of global pharmaceutical companies moving away from China is likely to accelerate," adding, "Korea is expected to benefit from the restructuring of the global bio supply chain based on its excellent bio production capabilities and stable political standing."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














