Development of Solar Hydrogen Production Technology Using Furfural and Silicon Photoelectrodes
Achieves Nearly Four Times the Commercialization Standard in Production Rate; Published in Nature Communications
A technology that produces hydrogen using sugarcane bagasse and sunlight has been developed.
The research team led by Professors Jang Jiuk and Seo Gwanyong from the Department of Energy and Chemical Engineering at UNIST, together with Professor Cho Seungho's team from the Department of Materials Science and Engineering, has developed a technology to produce hydrogen from biomass derived from sugarcane bagasse and a silicon photoelectrode.
 Research team (from top right, counterclockwise): Professor Jang Jiuk, Professor Seo Gwanyong, Researcher Lee Myeonghyeon, Dr. Jin Wonju, Dr. Jang Wonsik. Provided by UNIST
Research team (from top right, counterclockwise): Professor Jang Jiuk, Professor Seo Gwanyong, Researcher Lee Myeonghyeon, Dr. Jin Wonju, Dr. Jang Wonsik. Provided by UNIST
This technology produces hydrogen using only sunlight, without any external power supply, and its hydrogen production rate is four times higher than the commercialization standard set by the U.S. Department of Energy.
Hydrogen does not emit greenhouse gases when burned and can store 2.7 times more energy per unit weight than gasoline, making it a next-generation fuel. However, most hydrogen currently produced is extracted from natural gas, a process that generates significant amounts of carbon dioxide.
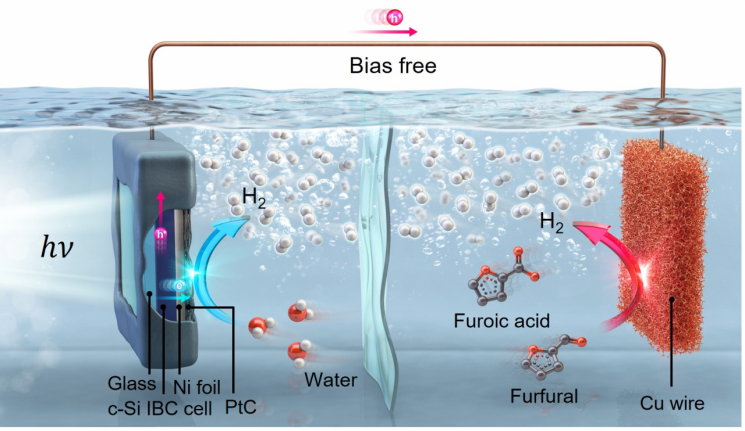
The research team developed a photoelectrochemical system for hydrogen production with zero carbon dioxide emissions by using furfural derived from sugarcane bagasse. As furfural is oxidized at the copper electrode, hydrogen is produced, and the remaining substance is converted into furoic acid, a high-value-added material.
In this system, hydrogen is produced at both electrodes. At the opposite electrode, the silicon photoelectrode, water is decomposed to produce hydrogen as well. This enables a theoretical production rate twice that of conventional photoelectrochemical systems, and in practice, a rate of 1.4 mmol/cm²·h was achieved. This is nearly four times the commercialization standard of 0.36 mmol/cm²·h set by the U.S. Department of Energy (DOE).
In this system, hydrogen production begins when the photoelectrode absorbs sunlight and generates electrons. While crystalline silicon photoelectrodes are advantageous for hydrogen production because they can generate many electrons, the voltage produced is low, making it difficult to trigger hydrogen production reactions without an external power source.
The research team addressed this issue by inducing the oxidation of furfural at the opposite electrode to balance the system voltage. This preserved the high photocurrent density?a strength of crystalline silicon photoelectrode materials?while reducing the overall system voltage burden, allowing hydrogen production without external power. Photocurrent density is an indicator of the flow of electrons per unit area and is directly related to the hydrogen production rate.
Additionally, the system adopted a back-contact (IBC) structure to reduce voltage loss inside the photoelectrode and ensured long-term stability by encapsulating the photoelectrode with nickel foil and a glass layer to protect it from electrolytes.
The study also confirmed that the structure in which the silicon photoelectrode is submerged in water provides a self-cooling effect, making it superior in efficiency and stability compared to external-coupled structures. An external-coupled structure refers to a configuration where the cell that generates electricity for water splitting and the electrolyzer that produces hydrogen are separated.
Professor Jang Jiuk said, "This technology achieves a solar-based hydrogen production rate four times higher than the commercialization standard set by the U.S. Department of Energy, which can play a key role in enhancing the economic feasibility of solar hydrogen and securing price competitiveness against fossil fuel-based hydrogen."
This research was supported by the Ministry of Trade, Industry and Energy, the Korea Institute of Energy Technology Evaluation and Planning's Global Energy Talent Training Program, and was published on March 19 in the world-renowned scientific journal 'Nature Communications.'
UNIST researchers Ko Myohwa, Lee Myeonghyeon, Kim Taehyeon, Jin Wonju, and Jang Wonsik participated as co-first authors.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














