Aiming to Enter 100 Countries by 2027
Daewoong Pharmaceutical has officially launched Pexuclu in India, intensifying its pursuit of the world's fourth-largest anti-ulcer drug market.
Daewoong Pharmaceutical announced on the 7th that it has officially launched Pexuclu in the Indian market. This is the first time a domestically produced P-CAB drug has been introduced in India. According to 2023 data from the global market research firm IMS, India ranks as the fourth-largest anti-ulcer drug market worldwide, following China, the United States, and Japan. The annual market size exceeds 1.4 trillion KRW.
Previously, Daewoong Pharmaceutical selected India as a key global hub for Pexuclu and signed a technology transfer agreement with Sun Pharma, India's largest pharmaceutical company, in December 2023. Since then, the company has rapidly proceeded from product approval application to launch, successfully completing its local market entry.
Pexuclu is a third-generation treatment for gastroesophageal reflux disease (GERD) launched by Daewoong Pharmaceutical in 2022. It improves upon the drawbacks of existing PPI drugs (proton pump inhibitors), such as slow onset of action, short half-life, and the need for pre-meal administration.
Sun Pharma conducted Phase 3 clinical trials on patients with erosive gastroesophageal reflux disease recruited locally in India. The trials demonstrated non-inferiority and significant improvement in primary symptoms compared to the PPI drug esomeprazole at 8 and 4 weeks of treatment, leading to product approval from the Central Drugs Standard Control Organization of India.
According to the clinical results, the healing rate of erosive gastroesophageal reflux disease patients at 8 weeks was 95.05% in the Pexuclu group and 92.93% in the control group receiving esomeprazole. The difference in treatment rates between the two groups was 2.12% (95% confidence interval -4.47%, 8.71%), indicating that once-daily administration of Pexuclu 40mg was non-inferior to esomeprazole 40mg. This successfully replicated results similar to those in Korea within the Indian population.
The improvement effects on the main symptoms of erosive gastroesophageal reflux disease, such as heartburn and acid reflux, were also confirmed. When examining the proportion of days without heartburn and acid reflux symptoms during daytime, nighttime, and over 24 hours, no statistically significant difference was found between the two groups regarding symptom improvement. Notably, at 8 weeks, the proportion of nights without heartburn and acid reflux symptoms was 70.28% and 73.29% in the Pexuclu group, respectively, which were 10.5% and 13.8% higher than those in the esomeprazole group.
Additionally, through the CGI-I (Clinical Global Impression of Improvement) scale, which evaluates symptom improvement, the proportion of patients assessed as "very much improved" or "much improved" at the end of 8 weeks was 96.2% in the Pexuclu group and 87.8% in the esomeprazole group, confirming the overall clinical symptom improvement with Pexuclu.
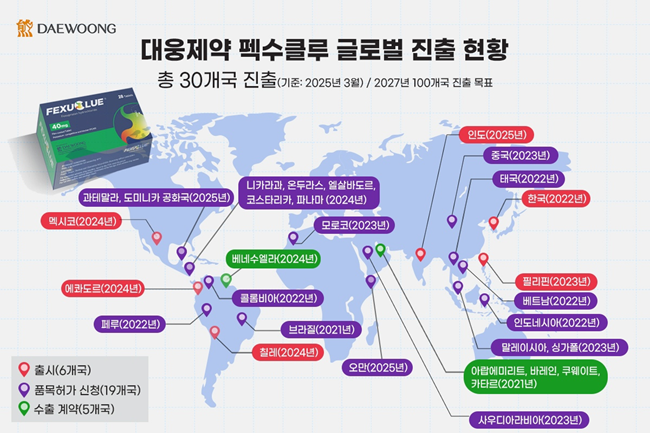
Meanwhile, Pexuclu surpassed 100 billion KRW in annual sales last year, its third year since launch, establishing itself as a blockbuster drug. With this launch in India, it is now sold in six countries including Korea, Mexico, Chile, Ecuador, and the Philippines. Additionally, marketing authorization applications have been submitted in 19 countries, and export contracts have been signed with five countries, expanding its presence to a total of 30 countries. Daewoong Pharmaceutical is accelerating its global expansion with a goal to enter 100 countries by 2027.
Park Sung-soo, CEO of Daewoong Pharmaceutical, said, "Through this launch, I am confident that Pexuclu will establish itself as a groundbreaking treatment option for patients with gastroesophageal reflux disease in India, the world's fourth-largest anti-ulcer drug market. Based on the differentiated advantages of Pexuclu compared to existing GERD treatments, we will further accelerate our efforts to enter 100 countries by 2027 and achieve our vision of 1 trillion KRW in sales per product by 2030."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














