Despite the Surge of Domestic and Global Products,
Institutional Frameworks and Public Perception Still Have a Long Way to Go
A veterans hospital in the United States. A retired soldier puts on a virtual reality (VR) headset and enters a virtual battlefield. Gunshots ring out, and amidst dusty bunkers and helicopter sounds, he moves slowly and confronts scenes from his memories. This is a program called 'Bravemind,' developed jointly by the U.S. Department of Defense and the Institute for Creative Technologies (ICT) at the University of Southern California (USC). It recreates an environment similar to an actual battlefield in virtual space and is designed to alleviate symptoms such as avoidance behavior, insomnia, and flashbacks (the phenomenon of becoming immersed in intense memories triggered by certain cues in reality) by gradually helping patients regulate their responses under medical supervision.
 A child undergoing treatment using EndeavorRx. Improving the child's attention through gaming. Akili Interactive homepage.
A child undergoing treatment using EndeavorRx. Improving the child's attention through gaming. Akili Interactive homepage.
Some elderly care facilities in the U.S. have installed customized game programs to prevent cognitive decline. Various games that stimulate memory, attention, and spatial cognition are provided, and the content automatically adjusts according to the user's reaction speed and performance accuracy. These games are not mere entertainment but digital therapeutics aimed at maintaining brain health in old age and delaying early symptoms of dementia. The number of elderly care facilities installing these game programs is gradually increasing.
In this way, digital technology is spreading worldwide as a tool that goes beyond simple health management assistance to actual treatment. The collective term for this concept is Digital Therapeutics (DTx).
DTx Established as an Actual Treatment 'Tool'... First Approval Worldwide by the U.S. in 2017
After the U.S. Food and Drug Administration (FDA) first approved the digital therapeutic 'reSET' in 2017, digital therapeutics have gradually appeared in South Korea as well. As of 2023, five companies including AimMed, Welt, NewNaps, Share & Service, and NewLive have received approval from the Ministry of Food and Drug Safety (MFDS) and their products have begun to be prescribed in actual hospitals.
DTx is a software-based treatment method. It uses mobile applications (apps) or wearable devices to analyze patient behavior data and provide tailored therapeutic interventions accordingly. Unlike unilateral instructions such as medication guidance, it is a digital interactive therapy that operates based on patient responses. It attracts attention for having fewer side effects than traditional drug treatments and for encouraging patients' voluntary participation.
It is especially meaningful for diseases where daily self-management is important, such as mental health conditions, diabetes, or hypertension. Even if patients visit hospitals for consultations or prescriptions, most of the time afterward must be managed alone. In this context, digital therapeutics can serve as a tool to fill 'that gap time.'
A representative example is 'EndeavourRx,' an attention deficit hyperactivity disorder (ADHD) treatment game developed by Akili Interactive in the U.S. in 2020. When a guardian installs and verifies the app, children train their attention by avoiding flying obstacles. Clinical results showed actual improvement in concentration after a program of 25 minutes per day, five days a week, for four weeks, but commercial success did not follow.
On the other hand, cases with institutional support have succeeded in market establishment. The UK's insomnia treatment 'Sleepio,' developed in 2018, became an official digital therapeutic option supported partially by the National Health Service (NHS) since 2022.
MFDS Grants First DTx Approval to AimMed's 'Somz' in March 2023
In South Korea, actual prescriptions and product launches have also begun. AimMed received MFDS product approval in February 2023 for 'Somz,' an insomnia treatment. It is an app-based program that helps improve sleep habits and behavioral interventions over 6 to 9 weeks, marking the first digital therapeutic approval case in Korea.
'Somz' is an app-based program specialized for sleep disorders. Users record daily sleep logs and undergo sleep hygiene education and assignments designed based on cognitive behavioral therapy for insomnia (CBT-I) principles. Daytime activities and emotional states are also monitored to induce sleep improvement.
NewNaps' 'VIVID Brain' is a training program for patients with visual field defects after stroke. It activates the brain's compensatory pathways by repeatedly performing game-like training that responds to visual stimuli. The stages adjust according to reaction speed and accuracy, enhancing therapeutic effects rather than merely repeating simple stimuli.
Share & Service's 'EasyBreath' provides respiratory rehabilitation training for patients with chronic respiratory diseases experiencing breathing difficulties. Users measure their breathing patterns through a device linked to a smartphone and perform breathing exercises at a set tempo to improve cardiopulmonary function.
Additionally, Welt's 'SleepQ' received MFDS approval in April 2023, and NewLive's tinnitus treatment 'SoriCLEAR' was approved as the fifth digital therapeutic in Korea in February 2025.
However, not all these attempts lead to success. The U.S. company Pear Therapeutics' 'reSET,' initially spotlighted as the first DTx, was an app for opioid addiction treatment but failed to enter health insurance coverage and the developer went bankrupt in 2023.
"Flexible Institutional Application and Health Insurance Reimbursement Needed"
Experts point out several issues. First is the flexibility of institutional application. Professor Cho Cheol-hyun of Korea University Department of Psychiatry said, "One of the main contents of DTx is games, but DTx games are not as fun as other games. Due to concerns about game addiction, there are restrictions from the development stage," adding, "If it is not truly pathological addiction, flexible application is needed to make them enjoyable."
Professor Nam Hyun-woo of Dongduk Women's University Department of Computer Science expressed concerns about social perception barriers. "Since gaming addiction was registered as a disease code, DTx-based therapeutic games often face rejection from parents and the public," he said, emphasizing, "It is necessary to distinguish therapeutic games from general games and approach them flexibly institutionally."
The vulnerability of the institutional foundation is also a problem. Professor Cho said, "DTx does not have a clear control group like new drugs, and it is difficult to apply existing clinical trial frameworks as is," diagnosing, "Although effective, it is hard to say the effects are 'dramatic' statistically, which prolongs the review process and creates uncertainty."
Both experts commonly advise that "a regulatory system different from existing medical devices is needed." They argue that separate pathways should be established to recognize innovative technologies, such as special evaluations for new medical technologies, differential application of insurance reimbursement, and technical special listing.
Professor Nam said, "Affordable medical insurance reimbursement that anyone can use is necessary. If safe DTx can be used for treatment at a low cost, many patients will seek it," adding, "It may be possible to differentiate insurance reimbursement methods by separating cases where medical staff perform direct medical acts and cases where patients are educated to use it themselves."
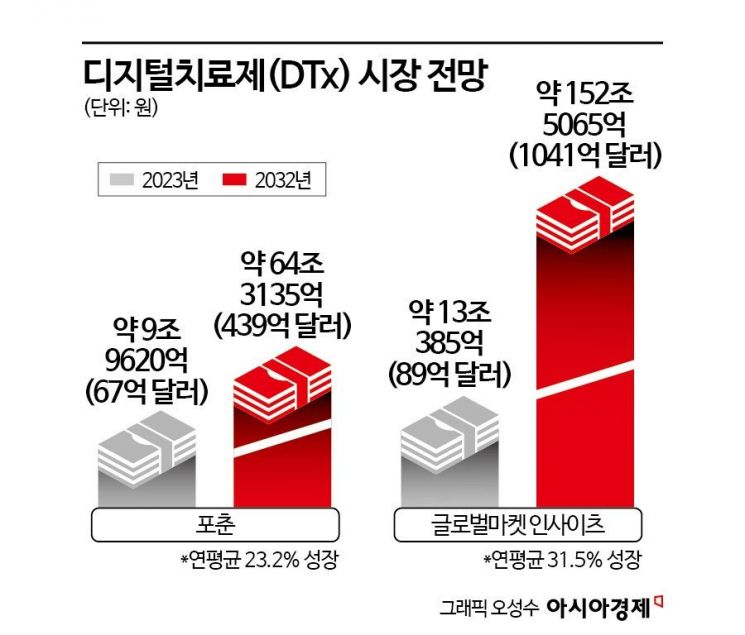
The digital therapeutics market still lacks a fully established institutional foundation, and core structures such as insurance entry are in the formation stage, making it difficult to accurately estimate market size. Because of this, there is a wide range of forecasts among market research organizations.
Nevertheless, most institutions agree that digital therapeutics will grow rapidly in the mid to long term. Fortune estimated that the global market, which was about $6.8 billion (approximately 9.962 trillion KRW) in 2023, will grow to about $43.9 billion (approximately 64.3135 trillion KRW) by 2032. Global Market Insights projected expansion from $8.9 billion (approximately 13.0385 trillion KRW) to $104.1 billion (approximately 152.5065 trillion KRW) during the same period. Although the forecast ranges differ, both institutions present high growth rates ranging from the high 20% to low 30% annually.
Professor Cho said, "The MFDS is relatively fast and flexible, but the recently enacted Digital Medical Device Act is excessively regulated at the level of traditional medical devices," adding, "Establishing a separate management system tailored to the characteristics of DTx is rather the way to improve institutional efficiency."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.