The 6th Biohealth Innovation Committee Held
44,000 Talents Nurtured Last Year, Double the Target
Discussion on Improving 'Thorns Under the Fingernail' Regulations
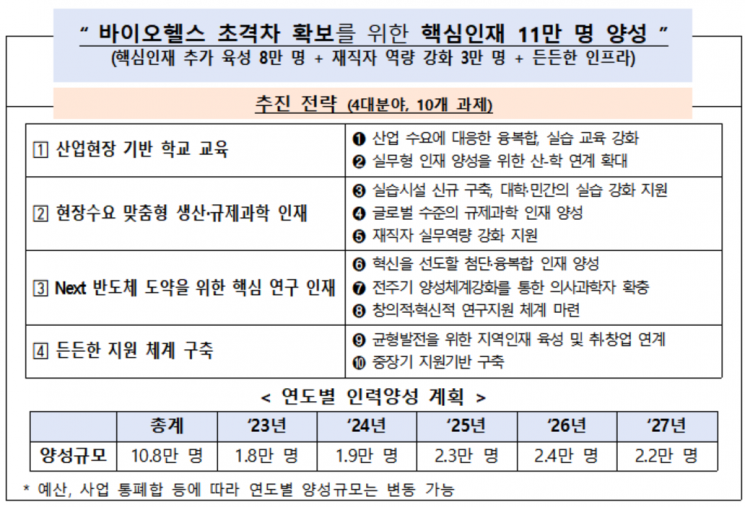
The government plans to nurture 27,000 talents in the biohealth (life sciences) sector this year. The policy aims to cultivate talents tailored to the demands of the field, such as companies, to resolve the mismatch between talent supply and demand. Additionally, even for drugs subject to administrative sanctions such as fines, the drug price may be increased in exceptional cases where a shortage of supply could disrupt patient care.
 Kim Young-tae, director of Seoul National University Hospital, is speaking at the '5th Biohealth Innovation Committee' held on December 24 last year at the Government Seoul Office in Jongno-gu, Seoul. Photo by Yonhap News
Kim Young-tae, director of Seoul National University Hospital, is speaking at the '5th Biohealth Innovation Committee' held on December 24 last year at the Government Seoul Office in Jongno-gu, Seoul. Photo by Yonhap News
On the 25th, the government held the '6th Biohealth Innovation Committee' meeting to review the implementation status of talent development measures to strengthen industrial capabilities in the biohealth sector and to discuss agendas for improving unreasonable regulations in the industry field.
First, this year, 26,900 talents will be nurtured through 78 biohealth projects. The plan is to cultivate a total of 108,000 talents, including physician-scientists and master's and doctoral graduates in advanced technology fields, by 2027. This year, especially, the number of talents will be increased through industry-academia cooperative education.
Furthermore, to resolve the frequently raised mismatch between talent supply and demand in the biohealth industry, the government decided to nurture high-quality talents demanded by the field, such as talents customized to corporate needs. To this end, the '2025 Biohealth Talent Development Project Guide' will be published in May, and policy research will also be promoted.
Previously, the government planned to nurture 22,100 talents through 81 projects across 9 ministries last year, but actually nurtured 44,800 talents, twice the planned number.
At the meeting, the government also reviewed the regulatory innovation achievements in the biohealth sector for the first quarter of this year. It examined 32 improvement items that had been reviewed by ministries since the previous meeting and selected seven 'killer regulations' considered as 'thorns under the fingernail' in the biohealth industry to discuss improvement measures.
Accordingly, the government will revise the medical device software validation guidelines to comply with international standards and plans to establish guidelines for clinical evaluation of medical devices.
In response to field demands, the government raised the upper limit of production cost compensation for plasma fractionation products (medicines that extract and purify necessary components without altering specific proteins in the blood) listed in health insurance and improved related standards so that drugs subject to administrative sanctions such as fines can be eligible for drug price ceiling adjustments to protect patients' treatment rights. To alleviate the burden of high-risk clinical research in advanced regenerative medicine, an exception criterion for safety and efficacy evidence in clinical research was also newly established.
Kim Young-tae, Vice Chairman of the Biohealth Innovation Committee and Director of Seoul National University Hospital, said, "We will periodically review and strive at the Biohealth Innovation Committee level to ensure that the discussions are reflected in government policies and lead to clear results."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














