Photoelectrochemical Synthesis of Ammonia Without Carbon Emissions
World's Highest Solar Ammonia Production Efficiency and Rate Achieved
A new technology has been developed to produce ammonia and glycolic acid, a cosmetic ingredient, from wastewater and waste plastics.
The research team led by Professors Cho Seungho and Song Myunghoon from the Department of Materials Science and Engineering at UNIST has developed a technology that can produce ammonia without emitting carbon dioxide by using solar electricity.
 Professor Cho Seungho
Professor Cho Seungho
This technology converts nitrate pollutants in wastewater into ammonia through an electrochemical reaction. During the ammonia production process, glycolic acid derived from waste plastics is also generated. This enables the reduction of carbon emissions and the processing of waste plastics to produce high value-added substances.
 Professor Song Myunghun
Professor Song Myunghun
Ammonia is the most widely produced inorganic compound in the world after sulfuric acid, but the carbon dioxide emitted during its production accounts for as much as 1.4% of total global carbon dioxide emissions. This is why the development of eco-friendly ammonia production technology to replace the century-old Haber-Bosch process is necessary.
The joint research team developed a photoelectrochemical system that synthesizes ammonia at the cathode and glycolic acid at the anode using solar electricity.
Nitrite (NO2-) in wastewater is reduced to ammonia at the cathode by the energy from solar electricity. The electrochemical system involves paired reactions, and as a result, at the anode, ethylene glycol is oxidized to glycolic acid. Ethylene glycol is a raw material extracted from waste plastics.
The energy efficiency of this system reached 52.3% (cathode only), the highest ever reported. The rate of ammonia production also reached 146 μmol/cm2h, surpassing the U.S. Department of Energy's commercialization standard for solar ammonia production of 58.72 μmol/cm2h. This is more than a 46% improvement over the previous record.
The research team developed a catalyst (RuCo-NT/CF) that selectively reduces nitrite in wastewater, enabling this high-efficiency system. In wastewater, nitrate (NO3-) and nitrite are mixed, but producing ammonia from nitrite is much faster and requires less energy. In addition, the paired reaction in the system was chosen to generate glycolic acid instead of the energy-intensive oxygen evolution reaction, further reducing the required electrical energy.
The perovskite solar cell that supplies electrical energy was also designed to have high photocurrent density and durability. The higher the photocurrent density, the faster the ammonia production rate.
Professor Song Myunghoon explained, "This is a meaningful study as it demonstrates the potential of electrochemical ammonia production without carbon dioxide emissions using perovskite solar cells, which have higher efficiency than commercial silicon solar cells."
The research team also verified the commercial viability of the technology. The electrochemical system using an electrolyte simulating low-level radioactive wastewater and PET bottle extracts achieved a solar ammonia production rate of 114 μmol/cm2h.
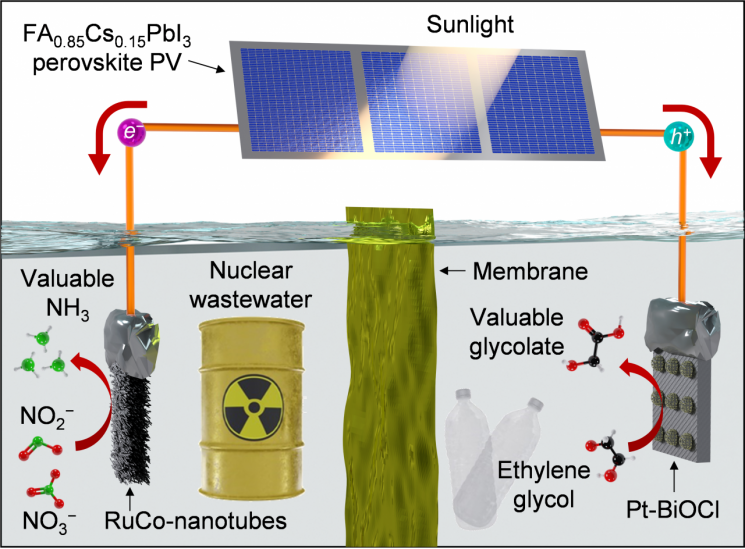 Electrochemical System for Ammonia and Glycolic Acid Production Using Wastewater and PET Bottle Extracts.
Electrochemical System for Ammonia and Glycolic Acid Production Using Wastewater and PET Bottle Extracts.
Professor Cho Seungho stated, "This study presents a sustainable, carbon-neutral energy solution by simultaneously producing green ammonia and high value-added glycolic acid from solar energy and waste."
This research was jointly led by researchers Jang Wonsik, Kim Jongkyung, and Kim Hyesung as co-first authors, and was supported by the Basic Research Laboratory Program of the Ministry of Science and ICT.
The results were published on February 19 in 'Nano Letters,' a prestigious international journal in the field of nanoscience.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














