Delisting Crisis in May 2019, Trading Resumed in October 2022
FDA Approval for Osteoarthritis Cell Gene Therapy Expected by 2028
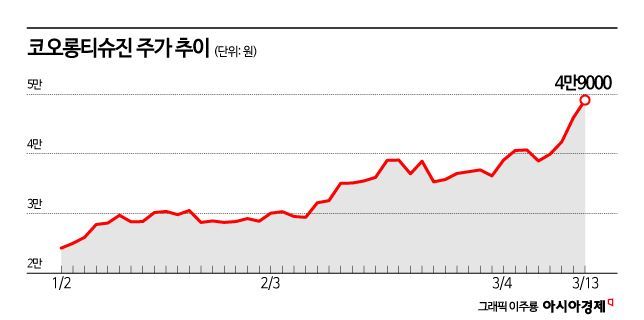
Market Cap Nears 4 Trillion Won... Stock Price Soars 453% in Two Years
In May 2019, Kolon TissueGene, which faced the risk of delisting after the Ministry of Food and Drug Safety revoked the approval of its osteoarthritis cell gene therapy product (Invossa-K), made a spectacular comeback. The Korea Exchange KOSDAQ Market Division's Corporate Review Committee decided to maintain its listing after deliberation, and since resuming stock trading on October 25, 2022, the company's value has steadily increased. Nomun Jong, CEO of Kolon TissueGene, expressed confidence in obtaining approval for TG-C (formerly known as Invossa in Korea) in the United States.
According to the financial investment industry on the 14th, Kolon TissueGene's stock price rose 453.0% over about two years from January 2023. The stock price, which had been below 9,000 won, surged to 49,000 won. The market capitalization expanded to 3.995 trillion won. During the previous day's trading session, the stock price reached 52,000 won at one point, setting a new high since trading resumed.
Kolon TissueGene is developing TG-C, a gene therapy for osteoarthritis. It consists of two components: Component 1, made up of human cartilage cells, and Component 2, which contains the gene TGF-β1 that promotes cartilage cell proliferation and alleviates inflammation, a cause of joint pain. CEO Nom recently stated, "We expect to receive product approval by 2028."
Yeouido securities firms anticipate that if Kolon TissueGene obtains approval and sells the product as planned, it could achieve an operating profit exceeding 5 trillion won. Researcher Wi Haeju from Korea Investment & Securities explained, "If TG-C is approved as a disease-modifying osteoarthritis drug (DMOAD), sales could reach 7 to 8.2 billion USD," adding, "Assuming an operating margin of 55%, operating profit at 7 billion USD in sales would amount to approximately 5.5 trillion won."
The global number of osteoarthritis patients reached 600 million as of 2020. To date, there is no fundamental cure, and only temporary symptom relief is possible. The definitive treatment is artificial joint surgery, but joint replacement surgery carries infection risks and is difficult for patients with certain conditions. In the United States alone, annual medical expenses related to osteoarthritis amount to approximately 65 billion USD.
The U.S. Food and Drug Administration (FDA) places great importance on demonstrating pain relief when approving new osteoarthritis drugs. Additionally, if magnetic resonance imaging (MRI) tests prove that the damaged cartilage has thickened, the drug can be designated as a disease-modifying osteoarthritis drug.
Previously, Kolon TissueGene launched a product called Invossa in Korea in 2017. However, it was revealed that the cells used in Component 2 were not the originally approved cartilage cells but cells derived from the kidney, leading the Korean Ministry of Food and Drug Safety to revoke the sales approval. The U.S. FDA imposed a clinical hold in May 2019 but approved the resumption of dosing procedures in April the following year. In July last year, 1,066 patients were enrolled. They are monitoring the progression of knee osteoarthritis after administering TG-C to the patients. The plan is to conduct the study for two years from the last patient's dosing date, followed by data analysis and submission of a product approval application to the FDA after the follow-up period ends.
Researcher Wi said, "Follow-up results are expected to be presented at the Osteoarthritis Research Society International (OARSI) conference starting on the 24th of next month," expressing hope that "efficacy and safety data will be further strengthened."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














