Recent Series of MASH Technology Returns and Clinical Trial Failures
Developing treatments for MASH (Metabolic Dysfunction-Associated Steatohepatitis), a market expected to grow to tens of trillions of won within a few years, is proving to be challenging. Recently, global pharmaceutical companies have been returning MASH treatment candidate substances one after another, and clinical trials have repeatedly ended in failure. However, as more treatments showing meaningful effects in clinical trials are emerging, there is also speculation that conquering MASH is not far off.
According to industry sources on the 11th, Yuhan Corporation recently received a notice of technology return from the German pharmaceutical company Boehringer Ingelheim for 'BI3006337 (YH25724),' a bispecific antibody targeting GLP-1 (glucagon-like peptide-1) and fibroblast growth factor 21 (FGF21). The reason for this technology return appears to be a strategic decision by Boehringer Ingelheim. Industry insiders believe there are no issues with the drug itself, including clinical results. Research results showing that the combination therapy of GLP-1 and FGF21 used in this candidate substance is effective in improving liver diseases are emerging one after another, and 'Akero Therapeutics,' which is developing a monotherapy targeting the hormone FGF21, has also reported positive clinical results. Junyoung Kim, a researcher at Meritz Securities, forecasted, "There is potential for additional technology transfers in the future."
This is the second time Yuhan Corporation has returned a MASH drug candidate. In October last year, Gilead terminated the technology transfer contract for a MASH drug candidate and returned the development rights to Yuhan Corporation. Dong-A Pharmaceutical’s drug development company Dong-A ST also exported 'Suganon' as a MASH treatment to the U.S. pharmaceutical company Tobira in 2016, but it was returned in 2017. Dong-A ST has stated that it secured efficacy through its own Phase 2 clinical trials. The final results of the global Phase 2 clinical trial are scheduled to be announced in the first half of this year.
Many global pharmaceutical and bio companies have entered the MASH treatment market, but clear results have been hard to come by. The diverse causes of MASH lead to varied drug responses among patients, increasing the difficulty of development. In December 2023, Pfizer decided not to proceed with Phase 3 clinical trials for the MASH treatment 'Danuglipron' after it showed a high incidence of adverse reactions in Phase 2b, with over 50% of patients discontinuing treatment. Gilead Sciences also halted development of 'Selonsertib.' Although Gilead conducted Phase 3 clinical trials using this candidate substance, it stopped development after failing to meet key evaluation criteria. AbbVie also abandoned development after failing to demonstrate meaningful effects in Phase 2 clinical trials of 'Acetrapib.'
MASH is a disease characterized by fatty liver and liver cell damage accompanied by fibrosis progression in severe cases, even without alcohol consumption. It mainly occurs in overweight, obese, diabetic, and high-cholesterol patients. The global prevalence is estimated at 2-4%. Until now, there has been no suitable treatment, and the main approach has been weight reduction through diet and exercise therapy and blood sugar control.
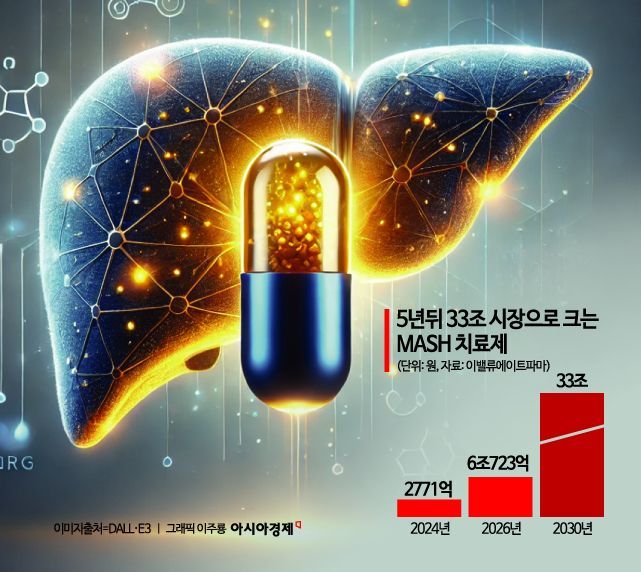
Then, in March last year, the U.S. pharmaceutical company Madrigal Pharmaceuticals succeeded for the first time in passing the U.S. Food and Drug Administration (FDA) approval hurdle with 'Rezdiffra.' Despite low response rates and side effects, this drug recorded sales of $180.1 million (approximately 260 billion won) last year, with 11,800 patients using it. According to the pharmaceutical market research firm EvaluatePharma, the MASH treatment market size is expected to grow from 277.1 billion won last year to 6.0723 trillion won by 2026. It is projected to expand to 33 trillion won by 2030.
Hyemin Heo, a researcher at Kiwoom Securities, said, "(The MASH treatment market) is seeing more sophisticated data than Madrigal’s product, so we expect that latecomers will be able to overcome hurdles more easily." She added, "Especially, data show that combination therapies using GLP-1 and glucagon (GCG) have greater effects on improving fatty liver than monotherapies." Domestic companies such as Yuhan Corporation, Hanmi Pharmaceutical, and Dong-A ST are developing MASH treatments using combination therapies.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














