Development of a Redox Mediator with High Stability in Reactive Oxygen Environments by UNIST and Ajou University
Lithium-Air Battery Lifespan and Energy Efficiency Improved... Published in Advanced Materials
An additive that can improve the lifespan and efficiency of lithium-air batteries, which use air as the electrode, has been developed.
It is expected to accelerate the commercialization of lithium-air batteries, which have a capacity up to five times greater than conventional lithium-ion batteries.
On the 10th, Professor Kwak Won-jin’s team at UNIST announced that they, in collaboration with Professor Seo Sung-eun’s team from Ajou University’s Department of Chemistry and Professor Shuming Chen’s team from Oberlin College in the United States, have developed a ‘redox mediator’ for lithium-air batteries.
 Research team. (From right) Professor Gwak Won-jin, Researcher Lee Hyun-wook, Researcher Yoon Hong-bin. Provided by UNIST
Research team. (From right) Professor Gwak Won-jin, Researcher Lee Hyun-wook, Researcher Yoon Hong-bin. Provided by UNIST
The redox mediator is an additive that accounts for only 5% of the battery electrolyte weight but determines the energy efficiency and lifespan of lithium-air batteries. Lithium-air batteries require a high voltage to charge, and the redox mediator is the substance that lowers the voltage applied during charging. Using a redox mediator allows the battery to be charged at a lower voltage, increasing energy efficiency and reducing the overload on the battery, thereby extending its lifespan.
The research team developed a redox mediator that does not react easily with reactive oxygen species. Since lithium-air batteries use oxygen as the electrode, a large amount of reactive oxygen species is generated inside. These reactive oxygen species, especially singlet oxygen, react with the redox mediator, preventing it from performing its original function.
The developed redox mediator, BAC (7,7′-bi-7-azabicyclo[2.2.1]heptane), maintained the battery charging voltage at about 3.5V before and after exposure to singlet oxygen, and demonstrated excellent stability and reversibility with oxygen release rates of 82% and 79% during charging, respectively.
This contrasts with other redox mediators, which show a significant increase in charging voltage and a decrease in oxygen generation by more than 50% after exposure to singlet oxygen. A decrease in oxygen generation indicates that the redox mediator reacted with singlet oxygen and other species, exhibiting irreversibility by not returning to its original state.
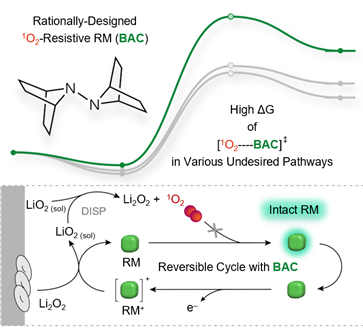 Schematic diagram of the developed redox mediator (BAC) showing high chemical stability toward singlet oxygen and the resulting reversible reaction in lithium-air batteries.
Schematic diagram of the developed redox mediator (BAC) showing high chemical stability toward singlet oxygen and the resulting reversible reaction in lithium-air batteries.
First author Researcher Lee Hyun-wook explained, “We were able to develop such a redox mediator through a design method analyzing the molecule’s stereostructure.” The newly developed redox mediator has one hydrogen attached to the ‘alpha carbon,’ and according to Bredt’s rule, which describes the structure of synthesizable organic compounds, this molecular structure has low reactivity with singlet oxygen. The alpha carbon refers to the carbon atom directly attached before the chemical functional group.
Professor Kwak Won-jin stated, “Lithium-air batteries exhibit various side reactions caused by reactive oxygen species, and controlling these is essential to improve the system’s technological level. The electrolyte additive design process used in this study is expected to enhance lithium-air battery technology and also be applied to the development of various catalysts.”
This research was published online on January 3, 2025, in the world-renowned international journal Advanced Materials and is scheduled for formal publication soon.
The research was supported by the Nano and Materials Technology Development Project of the Ministry of Science and ICT.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














