Utilizing Molten Metal Catalysts Containing Selenium
Methane-to-Hydrogen Conversion Rate Improved by Up to 36.3%
Dr. Han Seung-joo's research team at the Korea Research Institute of Chemical Technology announced on the 9th that they have developed a technology to improve the efficiency of turquoise hydrogen production by utilizing molten metal catalysts (NiBi, CuBi) with added selenium (Se).
Turquoise hydrogen is produced through the thermal decomposition of methane (CH4) and does not emit carbon. It is an intermediate hydrogen between gray hydrogen, which can be mass-produced but emits a lot of carbon dioxide, and green hydrogen, which emits no carbon but has limitations in commercial application due to high costs.
 From the bottom left of the photo clockwise: first author Jooho Son, student researcher; corresponding author Seungjoo Han, senior researcher; co-author Donghyun Lee, student researcher; Kyungah Park, researcher. Provided by Korea Research Institute of Chemical Technology
From the bottom left of the photo clockwise: first author Jooho Son, student researcher; corresponding author Seungjoo Han, senior researcher; co-author Donghyun Lee, student researcher; Kyungah Park, researcher. Provided by Korea Research Institute of Chemical Technology
The solid carbon generated during the methane decomposition process can be industrially utilized in the form of graphite or carbon black, offering the advantage of economically using carbon without the need for separate storage or disposal.
Global market research firm VMR (Verified Market Reports) projected in a report published last December that the global market size for turquoise hydrogen production technology will grow from approximately $16.8 billion in 2023 to about $22.8 billion by 2030, at an average annual growth rate of 5.3%. The importance of turquoise hydrogen technology is increasing due to the growing global demand for clean energy and decarbonization efforts.
However, the methane decomposition method requires high temperatures, and when using solid catalysts, carbon deposits on the catalyst surface cause rapid deactivation, which has been a drawback.
To overcome the limitations of existing catalysts, the research team developed a ternary molten metal catalyst containing selenium that improves catalyst activity and bubble control performance. By using a liquid-state molten metal catalyst instead of conventional solid catalysts, carbon generated during methane decomposition can be easily separated, enabling stable reactions over long periods.
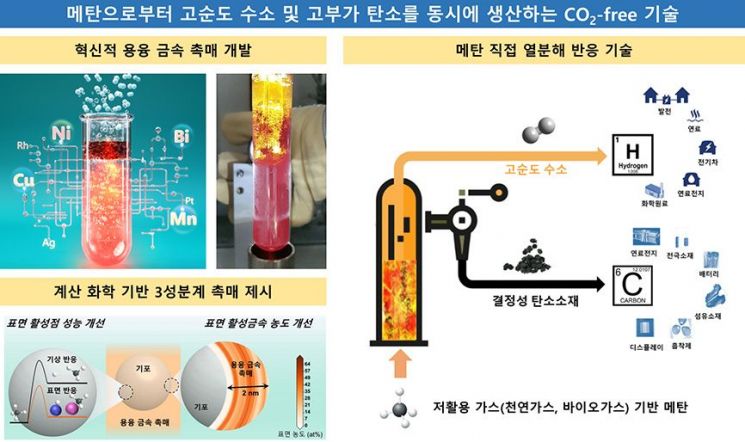 CO2-free technology that simultaneously produces high-purity hydrogen and high-value carbon from methane. Provided by Korea Research Institute of Chemical Technology
CO2-free technology that simultaneously produces high-purity hydrogen and high-value carbon from methane. Provided by Korea Research Institute of Chemical Technology
As the residence time of reactants within the catalyst increased, hydrogen productivity improved, and selenium reduced the activation energy for catalyst operation, promoting nickel surface exposure and enhancing the methane conversion efficiency of nickel active sites.
The ternary catalysts containing selenium (NiBiSe, CuBiSe) improved the methane-to-hydrogen conversion rates by 36.3% and 20.5%, respectively, compared to existing catalysts. In particular, the nickel-bismuth-selenium (NiBiSe) catalyst operated stably without performance degradation for over 100 hours of continuous reaction.
Dr. Han Seung-joo stated, "This research overcomes the limitations of existing turquoise hydrogen production technologies and is expected to make a significant contribution to achieving carbon neutrality." Lee Young-guk, President of the Korea Research Institute of Chemical Technology, also emphasized, "This technology, aimed at commercialization, will establish itself as a core technology for carbon-free turquoise hydrogen production."
The research results were published last December in the international journal in the materials and chemistry field, Applied Catalysis B: Environmental and Energy (IF=20.3). Dr. Han Seung-joo of the Korea Research Institute of Chemical Technology and Dr. Seo Jeong-cheol of the Korea Institute of Industrial Technology served as corresponding authors, with research student Son Ju-ho from the Korea Research Institute of Chemical Technology and Yonsei University participating as the first author.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














