Continuous Growth Through Global Market Expansion,
New U.S. Product Launches Announced for This Year
Expansion of Biosimilar Business Opportunities in the U.S.,
the World's Largest Market, Thanks to Favorable Policy Developments
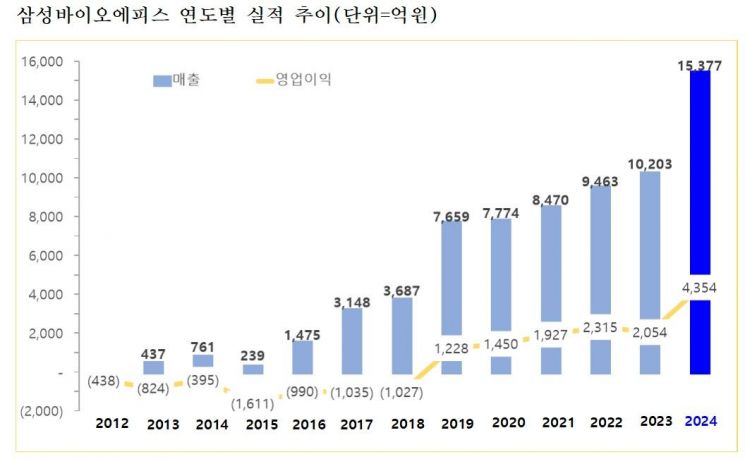
Samsung Bioepis, a developer of biosimilars (biopharmaceutical generics), achieved record-breaking results with sales of 1.5377 trillion won and operating profit of 435.4 billion won.
According to the earnings announcement by Samsung Biologics on January 23, Samsung Bioepis posted annual sales of 1.5377 trillion won and operating profit of 435.4 billion won last year. This represents a 51% increase in sales and a 112% increase in operating profit compared to its 2023 annual results (sales of 1.0203 trillion won and operating profit of 205.4 billion won), when it became the fastest domestic pharmaceutical developer to reach 1 trillion won in sales, marking its highest performance ever.
Samsung Bioepis stated, "We achieved strong growth through biosimilar approvals and sales performance in the global market," adding, "This year, we aim to continue this growth by expanding product sales, starting with a strategic focus on the U.S., the world's largest market."
Continuous Growth Through Global Market Expansion, New U.S. Product Launches Announced for This Year
Samsung Bioepis currently sells nine types of biosimilars in Korea, eight in Europe, and four in the United States, and is preparing to launch two new products in the U.S. within the year. Last year, thanks to partnerships with Biogen, Organon, and others, cumulative market sales for six biosimilar products (Enbrel, Humira, Remicade, Herceptin, Avastin, and Lucentis biosimilars) sold overseas reached $1.0906 billion (about 1.5671 trillion won) in the first three quarters, a 12% increase from the same period the previous year.
In addition, the Stelara biosimilar launched in Europe through Sandoz in July last year has achieved a market share of 43%, ranking first among biosimilars. The Soliris biosimilar, which Samsung Bioepis has been selling directly in Europe since July 2023, has also secured multiple tenders, further expanding the supply of all products in the global market.
Meanwhile, Samsung Bioepis has established partnerships to sell the Stelara biosimilar (Pyzchiva) with Sandoz and the Soliris biosimilar (Epysqli) with Teva in the U.S. market. Both products are expected to be launched in the U.S. within the first half of this year, following patent settlements with the original pharmaceutical companies. Samsung Bioepis plans to collaborate closely with partners that specialize in biosimilar sales to ensure timely product launches and secure an early lead in the market.
Last year, Samsung Bioepis achieved multiple product approvals both domestically and internationally, resulting in significant milestone revenue from partners holding overseas market rights. Milestone payments are compensation for R&D achievements and are recognized without additional costs, enabling simultaneous, high growth in both sales and operating profit. Last year, Samsung Bioepis obtained U.S. and European approvals for the Eylea biosimilar (Opuviz) and Stelara biosimilar (Pyzchiva), as well as U.S. approval for the Soliris biosimilar (Epysqli). Notably, the company received a total of three product approvals from the U.S. Food and Drug Administration (FDA) in a single year, once again demonstrating its world-class R&D and regulatory capabilities.
Expansion of Biosimilar Business Opportunities in the U.S., the World's Largest Market, Thanks to Favorable Policy Developments
The global biosimilar industry, which has grown mainly in Europe, is now entering a new phase of growth in the United States, the world’s largest pharmaceutical market, due to favorable policy changes and increased market competition. In particular, the U.S. FDA last year approved a record 18 products in a single year and is moving to revise interchangeability regulations, signaling efforts to revitalize the biosimilar market and ease regulations.
The Donald Trump administration in the United States made reducing national fiscal spending, including healthcare, a key policy pledge. In particular, biosimilars are expected to be adopted as a rational alternative to replace existing pharmaceuticals, given their availability and competitiveness. As a result, positive policy benefits are anticipated for the expansion of the biosimilar business.
Newly appointed CEO Kyeonga Kim pledged in her first New Year’s address to the company to "prepare for a second leap forward for Samsung Bioepis, emphasizing cooperation, passion, and an unceasing spirit of challenge." At the end of last year, Samsung Bioepis appointed Kyeonga Kim, who previously served as head of the development division, as the new CEO, establishing a new leadership structure. Kim is the first female professional executive CEO in Samsung Group’s history and joined Samsung Bioepis in 2015, gaining expertise across the entire biosimilar development process.
A Samsung Bioepis representative stated, "Kyeonga Kim is a leader with integrative leadership who can bring together top experts from all areas of the bio business," adding, "Under her new vision, we expect Samsung Bioepis will take another step forward as a global biotechnology company."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.


![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















