Professor Seohyeongtak's Team at Ajou University Developed
The research team at Ajou University has developed a two-dimensional nano new material that will enhance the utilization of future energy. This new material is expected to be applicable to both supercapacitors, a future energy storage technology, and anodes for hydrogen production.
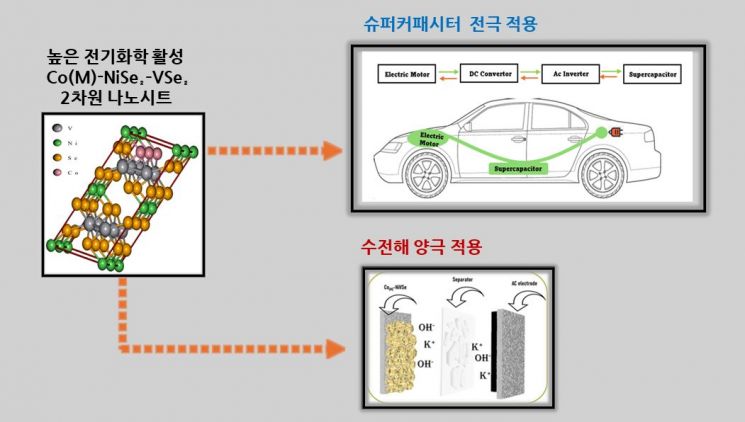
On the 26th, Ajou University announced that Professor Hyung-Tak Seo's team developed a quaternary two-dimensional nanosheet material of nickel, vanadium, and cobalt selenide (Co(M)-NiSe₂?VSe₂) with excellent electrochemical activity.
The related research was published online in the latest issue of the chemical engineering journal, Chemical Engineering Journal, under the title "Synergistic Effect of Donor Doping in Co(M)-NiSe₂?VSe₂ for Simultaneous Synthesis of Battery-Type Supercapacitors and Oxygen Evolution." Dr. Qadeer Akbar Sial, a full-time researcher at the Engineering Research Institute of Ajou University, participated as the first author in this study.
Supercapacitors are energy storage technologies that play a key role in sustainable future energy utilization. They are used in applications requiring rapid energy storage and high power delivery, such as electric vehicles, portable electronic devices, and renewable energy systems. Unlike conventional secondary batteries, supercapacitors can charge and discharge quickly, have a long lifespan, and offer high safety, making them a reliable and efficient energy storage solution. By supplying explosive power to complement batteries, supercapacitors optimize the performance of energy systems and are essential for the transition to renewable energy sources.
Producing "clean hydrogen," which is essential for using hydrogen as a core energy source, is possible through water electrolysis technology that efficiently generates hydrogen and oxygen by splitting water. This technology allows hydrogen energy to be obtained without emitting greenhouse gases such as carbon dioxide, utilizing renewable energy sources like solar, wind, hydro, and geothermal power to split water. To convert water into hydrogen and oxygen with high electrochemical efficiency during this process, electrodes with high electrochemical activity and long-term reaction stability are required.
The Ajou University research team developed a groundbreaking two-dimensional nano material by combining nickel, vanadium, and cobalt selenide into a flower-shaped nanosheet structure. The team confirmed that this material exhibits excellent performance simultaneously in hybrid supercapacitors and oxygen evolution reactions in water electrolysis.
The research team applied the material created through a cobalt-based electron donor doping strategy to supercapacitor devices and confirmed that it possesses a respectable power density at the basic material level. Furthermore, it achieved a high energy density of 38.73 Whkg?¹, representing an improvement of over 90% compared to existing commercial device characteristics. This material also demonstrated outstanding electrochemical stability by maintaining more than 90% of its capacitance after 10,000 charge-discharge cycles.
Additionally, the newly developed material was applied to the anode for the oxygen evolution reaction in water electrolysis, confirming its high performance as a water-splitting electrode material.
The research team identified that these electrochemical properties result from the synergistic effect of cobalt doping, an impurity addition technique. Density Functional Theory (DFT) calculations confirmed that cobalt doping alters the electronic band structure of the material and provides additional active electronic states for charge transfer, significantly enhancing the electrochemical activity of the material.
Professor Hyung-Tak Seo of the Department of Advanced New Materials Engineering, who led the research, explained, "The material developed this time not only has excellent electrochemical and catalytic performance but also exhibits high long-term reaction stability. It can be applied to next-generation energy storage systems for electric vehicles and grid systems that require high energy and power density as well as excellent cycling characteristics."
Professor Seo added, "In addition to energy storage, it is expected to be used as an electrochemical catalytic electrode for the oxygen evolution reaction (OER) in water electrolysis devices for hydrogen production. We will continue follow-up research for the commercialization of this material."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














