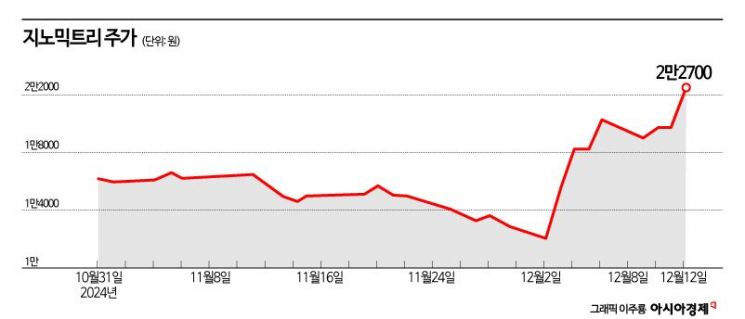
Stock Price Rises 74% This Month... Market Cap Surpasses 500 Billion KRW
Early Diagnosis Kit for Bladder Cancer Price Approved
Insurance Coverage Discussions Intensify
Since the 12·3 emergency martial law incident, volatility in the domestic stock market has increased, causing most stocks to fluctuate, while the corporate value of early cancer diagnosis company Genomictree has been rising sharply regardless.
According to the financial investment industry on the 12th, Genomictree's stock price has risen 73.5% this month. During the same period, the KOSDAQ index increased by 0.8%. Genomictree's market capitalization has exceeded 550 billion KRW.
Genomictree is developing technology that uses DNA methylation biomarkers abnormally occurring in cancer cells to enable early cancer diagnosis. It is conducting clinical trials to develop in vitro early cancer diagnosis products targeting solid cancers such as colorectal cancer, bladder cancer, and lung cancer. Major services include early colorectal cancer diagnosis using stool samples and early bladder cancer diagnosis using urine samples.
Earlier, in July this year, Genomictree submitted a manufacturing approval application for the in vitro diagnostic method for early bladder cancer diagnosis, 'EarlyTec,' to the Ministry of Food and Drug Safety. The majority of bladder cancer patients exhibit hematuria as a prodromal symptom. Patients suspected of bladder cancer with high-risk factors and hematuria must undergo cystoscopy, the standard diagnostic method. Due to the nature of cystoscopy, which often causes pain, many patients avoid the examination. There is an urgent need for an in vitro diagnostic method that can accurately identify patients likely to have bladder cancer at the hematuria patient screening stage.
Choi Jae-ho, a researcher at Hana Securities, explained, "EarlyTecB was designated as a breakthrough medical device by the U.S. Food and Drug Administration (FDA) in April," and "On the 3rd, the American Medical Association (AMA) and the Centers for Medicare & Medicaid Services (CMS) approved the final price of EarlyTecB at $192."
Approximately 82,000 people are diagnosed with bladder cancer annually in the United States alone. Considering that 3-17% of those tested for bladder cancer are confirmed cases, the number of suspected patients undergoing bladder cancer testing each year reaches about 800,000.
Han Jeyoon, a researcher at KB Securities, said, "With the price of EarlyTecB finalized, discussions on insurance coverage with private insurers will also begin in earnest," adding, "Since there are no clear competing products, it is expected to penetrate the U.S. market rapidly." He continued, "It is a situation where market dominance can be expected," and "Given the diagnostic kit's characteristics showing profitability above 40%, a steep increase in profits is anticipated as market penetration progresses."
Expectations for early colorectal cancer diagnosis products are also growing. Last month, Genomictree successfully completed the confirmatory clinical performance trial (NEXT-CRC) of the in vitro molecular diagnostic product EarlyTecC for early colorectal cancer diagnosis. EarlyTecC testing was conducted on 2,358 high-risk colorectal cancer patients aged 40 and above who were scheduled for colonoscopy. The test results were compared with colonoscopy and histopathological readings, statistically validating the effectiveness.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














