US Top 3 PBM Operate Listings in 6 Public and Private Insurance Areas
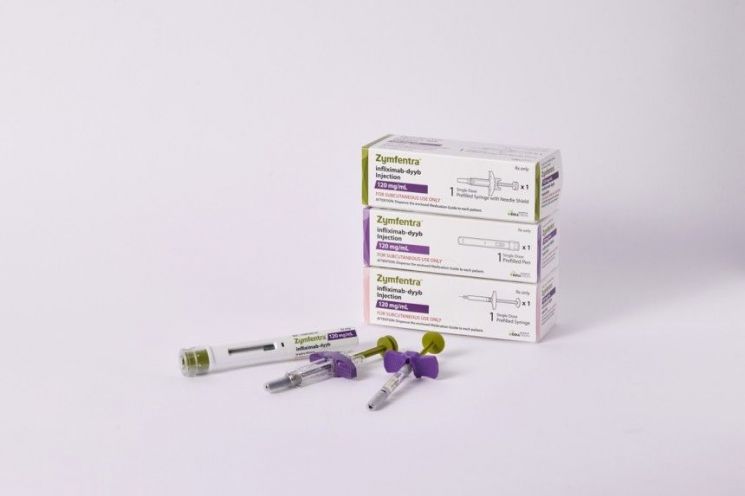
Celltrion's autoimmune disease treatment 'Jimpentra' will be listed in six public and private insurance sectors operated by the three major U.S. pharmacy benefit managers (PBMs).
On the 29th, Celltrion announced that it had signed a contract to list 'Jimpentra' in six public and private insurance sectors operated by the three major U.S. PBMs. In the U.S., PBMs act as intermediaries managing prescription drugs, and being listed on a PBM formulary means inclusion in the U.S. health insurance reimbursement system.
Jimpentra is a product developed by changing Celltrion's intravenous autoimmune disease treatment biosimilar 'Remsima' (generic name infliximab) into a subcutaneous injection form that can be self-administered. It was approved as a new drug by the U.S. Food and Drug Administration (FDA) in October last year and launched in March.
Celltrion negotiated the listing conditions for private insurance formularies with a group purchasing organization (GPO) representing one of the three major U.S. PBMs and finalized the agreement on the 25th (local time), signing the contract.
So far, contracts for Jimpentra have been completed with a total of 30 PBMs and insurers. Combined coverage of these accounts for over 90% of the U.S. insurance market.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














