Samsung Bioepis announced on the 30th that it presented an abstract of a research paper on Episcle at the European Society for Paediatric Nephrology (ESPN) Annual Meeting held in Valencia, Spain from the 24th to the 27th.
Episcle is a biosimilar of Soliris developed by Alexion in the United States. Soliris is used to treat rare diseases such as paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS). It is a blockbuster drug that recorded global sales of $3.145 billion (approximately 4.13 trillion KRW) last year. Noticing this growth trend, AstraZeneca acquired Alexion in 2020 for $39 billion (approximately 51 trillion KRW).
PNH is a disease caused by the destruction of red blood cells in the blood. It shows symptoms of hemoglobinuria and can cause complications such as acute renal failure, which can lead to death, making it a rare disease. However, the only current cure for PNH is bone marrow transplantation. Soliris is a drug that prevents the disease from worsening, so continuous administration is required. As a result, the annual treatment cost reaches about 400 million KRW domestically, placing a heavy financial burden on patients.
Samsung Bioepis explained that to address this unmet need, it launched the biosimilar Episcle at a reasonable price to improve patient access to ultra-high-priced drugs. Domestically, it was launched in April at a price of 2.51 million KRW per vial, about half the price of Soliris, which was 5.13 million KRW per vial. In response, Soliris also lowered its price by about 30% to 3.6 million KRW to secure price competitiveness. In Europe, Episcle was approved for the PNH indication in May last year, launched in July, and added the aHUS indication in March. In the United States, both indications were approved by the U.S. Food and Drug Administration (FDA).
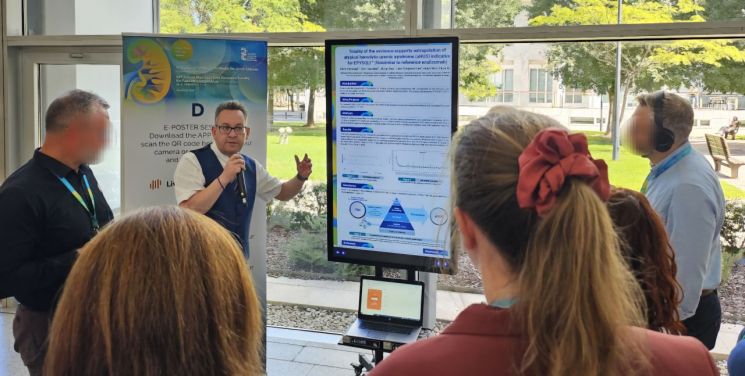 At the European Society for Paediatric Nephrology (ESPN) held in Valencia, Spain, Professor David Cabana from the Medical School of Newcastle University, UK, explained comprehensive evidence related to the extrapolation of the original drug's aHUS indication for Samsung Bioepis's rare disease treatment 'Episcle' to the conference participants on the 27th (local time).
At the European Society for Paediatric Nephrology (ESPN) held in Valencia, Spain, Professor David Cabana from the Medical School of Newcastle University, UK, explained comprehensive evidence related to the extrapolation of the original drug's aHUS indication for Samsung Bioepis's rare disease treatment 'Episcle' to the conference participants on the 27th (local time). [Photo by Samsung Bioepis]
Through this academic conference, Samsung Bioepis presented comprehensive evidence based on structural, physicochemical, biological characteristics, and nonclinical and clinical equivalence between Episcle and the original drug. According to the research results, Samsung Bioepis confirmed that extrapolation of the aHUS indication held by the original drug is possible, not only for PNH, for which the Episcle clinical trial (Phase 3) was conducted.
Kim Hye-jin, Medical Team Leader (Executive Director) at Samsung Bioepis, said, "We have proven that Episcle has equivalent efficacy and safety to the original drug, and we will continue to strive to provide a reasonable alternative for ultra-high-priced rare disease treatments to more patients.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















