Similar Efficacy and Safety Secured Compared to Existing Standard Treatment 'Tagrisso'
Improved Efficacy in Some High-Risk Patient Groups
FDA Approval Decision Expected This Week
J&J "Expecting 7 Trillion KRW Annual Sales from Combination Therapy"
Yuhan Corporation's lung cancer treatment, Lekraza, has demonstrated efficacy and safety comparable to existing global standard treatments, raising expectations for its leap to becoming a global blockbuster.
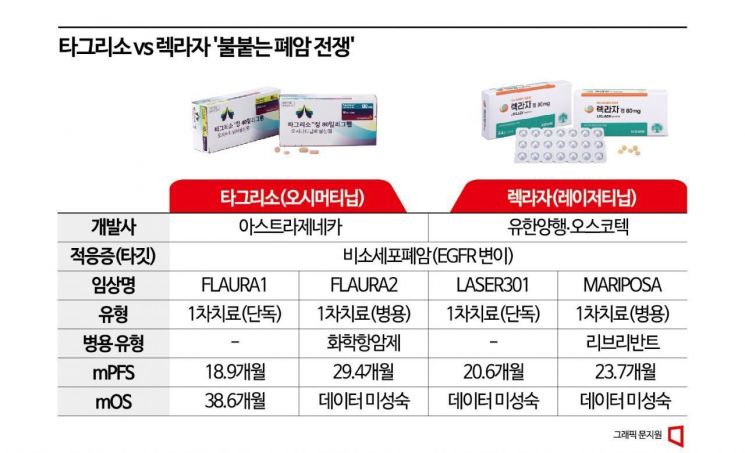
According to the recently released abstract book of the World Conference on Lung Cancer (WCLC 2024), Lekraza showed similar efficacy and safety in clinical trials compared to AstraZeneca (AZ)'s Tagrisso, the first recommended treatment for non-small cell lung cancer patients with epidermal growth factor receptor (EGFR) mutations. This study, led by Professor Sehoon Lee of the Hematology-Oncology Department at Samsung Seoul Hospital, will be presented on the second day, the 8th, of the WCLC 2024 event held from July 7 to 10 (local time) in San Diego, California, USA.
This study prospectively analyzed the 'Mariposa' clinical trial, a combination therapy trial conducted by global big pharma Johnson & Johnson (J&J) to use its targeted antibody treatment Rybrevant together with Lekraza. The Mariposa trial is designed to compare the efficacy and safety of the combination therapy against Tagrisso. However, J&J set both Tagrisso monotherapy and Lekraza monotherapy as control groups for efficacy comparison and analysis. Previously, Lekraza monotherapy was criticized for difficulty in comparison with the 'current' standard treatment because its approval clinical trial used the earlier generation drug Iressa as the control group, but this study overcomes that limitation.
As a result, the median progression-free survival (mPFS), a key indicator of anticancer drug efficacy measuring the period patients survive without cancer progression or recurrence after treatment, was 18.5 months for Lekraza monotherapy, slightly ahead of Tagrisso monotherapy's 16.6 months. The objective response rate (ORR), indicating the proportion of patients showing a positive response to the anticancer drug, was similar at 83% for Lekraza and 85% for Tagrisso. Safety profiles of the two drugs were also comparable, confirming that Lekraza demonstrates efficacy and safety not inferior to Tagrisso.
Notably, Lekraza showed potential for better efficacy in some high-risk patients, such as those with brain metastases or other special mutations. For patients with a history of brain metastasis, mPFS was 16.4 months for Lekraza compared to 13 months for Tagrisso, indicating more advanced efficacy for Lekraza. In patients with TP53 mutations, Lekraza also showed better efficacy with an mPFS of 14.6 months versus 12.9 months for Tagrisso.
This abstract release is especially anticipated ahead of the U.S. Food and Drug Administration (FDA) decision on July 22 (local time) regarding approval of the Lekraza-Rybrevant combination therapy. The combination therapy secured an mPFS approximately 5 months longer than Tagrisso monotherapy in the Mariposa trial. If the FDA approves this therapy, Lekraza will become the first anticancer drug developed in Korea to receive FDA approval. Previously, Hanmi Pharmaceutical's Rolontis was approved, but it is a drug for treating side effects of anticancer therapy rather than a fundamental anticancer agent, and HLB's Rivoceranib attempted approval but failed and is currently preparing for a re-challenge.
If approval is granted, J&J plans to launch Lekraza in the U.S. market as early as this year. J&J acquired the rights to Lekraza in December 2018 for $1.255 billion (approximately 1.7 trillion KRW) and expects the combination therapy to grow into a blockbuster generating annual sales of $5 billion (approximately 6.7725 trillion KRW).
Additionally, efforts are being accelerated for further development. Currently, Rybrevant, the combination partner of Lekraza, is only available as an intravenous formulation. Intravenous administration requires patients to stay in the hospital for extended periods, causing inconvenience, and medical institutions face burdens in maintaining various facilities. Therefore, J&J is developing a subcutaneous injection formulation of Rybrevant that can be administered directly in outpatient clinics and is currently pursuing FDA approval for this formulation.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














