Latecomer in CAR-T Following Multinational Pharma Lead
Low Recurrence Rate Raises Hopes to Replace Existing Treatments
Technology Transfer Planned After Midterm Results This Year
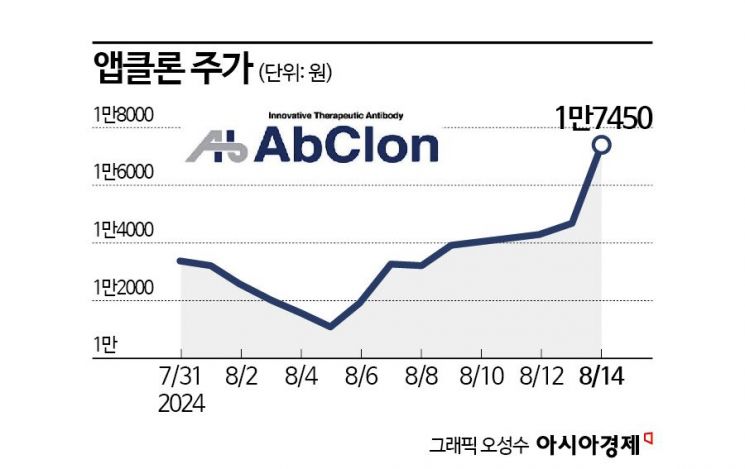
Appclon, developing next-generation antibody therapeutics, has recently seen a rebound in its stock price. There is growing anticipation for the development of new drugs that can overcome the shortcomings of existing chimeric antigen receptor T-cell (CAR-T) therapies, which currently generate annual sales in the hundreds of billions of Korean won.
According to the Korea Exchange on the 16th, Appclon's stock price rose 57.5% over seven trading days starting from the 6th. Considering that the KOSDAQ index increased by 12.4% during the same period, the excess return compared to the market reached 45.1 percentage points.
Appclon is a biotech company developing new drugs for various cancer types based on single monoclonal antibody and bispecific antibody platform technologies. AT101, developed using Appclon's CAR-T platform, is aimed at treating diffuse large B-cell lymphoma (DLBCL), which has the highest incidence rate among lymphomas.
CAR-T therapies are treatments that genetically engineer T cells extracted from a patient's blood to attack and kill targeted cancer cells. Globally, six CAR-T therapies are currently marketed, including Novartis's Kymriah and Gilead Sciences' Yescarta. In 2022, Kymriah and Yescarta recorded sales of $536 million and $1.16 billion, respectively. This explains why major pharmaceutical companies invest heavily in developing CAR-T therapies. However, existing treatments have been criticized for their high relapse rates.
Byungyong Oh, a researcher at Hanyang Securities, explained, "Kymriah and Yescarta face the issue that a significant number of patients relapse after more than six months post-treatment."
If the shortcomings of existing CAR-T therapies can be overcome, even latecomers have the opportunity to achieve annual sales in the hundreds of billions of Korean won. Appclon developed its therapy by removing immunogenicity based on a differentiated new antibody compared to Kymriah and Yescarta, which use the existing mouse-derived antibody (FMC63). Appclon is conducting a Phase 2 clinical trial involving 82 patients and plans to complete dosing for interim analysis this summer. If the trial proceeds as planned, results could be announced within this year.
At the American Society of Clinical Oncology (ASCO) held in June, Appclon disclosed follow-up data from its Phase 1 CAR-T clinical trial. This study confirmed treatment efficacy for about two years after Phase 1 administration. Among nine subjects who achieved complete remission (CR) in the Phase 1 evaluation, seven maintained complete remission for more than 12 months. The long-term complete remission rate reached 77.8%.
Researcher Oh said, "A paper on the low relapse rate was published in the journal 'Molecular Cancer' in December last year," adding, "Although Appclon is a latecomer, it is developing a CAR-T therapy with a lower relapse rate than Kymriah or Yescarta," expressing optimism.
Songhyeop Han, a researcher at Daishin Securities, analyzed, "Currently, over 60% of patients treated with CAR-T therapies experience non-response or relapse. Appclon demonstrated a complete remission (CR) rate of 75% and an objective response rate (ORR) of 92% in the Phase 1 trial of AT101, confirming that the therapeutic effect is maintained long-term." He added, "Interim results are expected to be announced in the second half of this year," and "the possibility of global technology export is increasing."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














