Medytox Smiles in First Trial... Second Trial Starts on the 26th of Next Month
Medytox and Daewoong Pharmaceutical Restructure Legal Teams Ahead of Second Trial
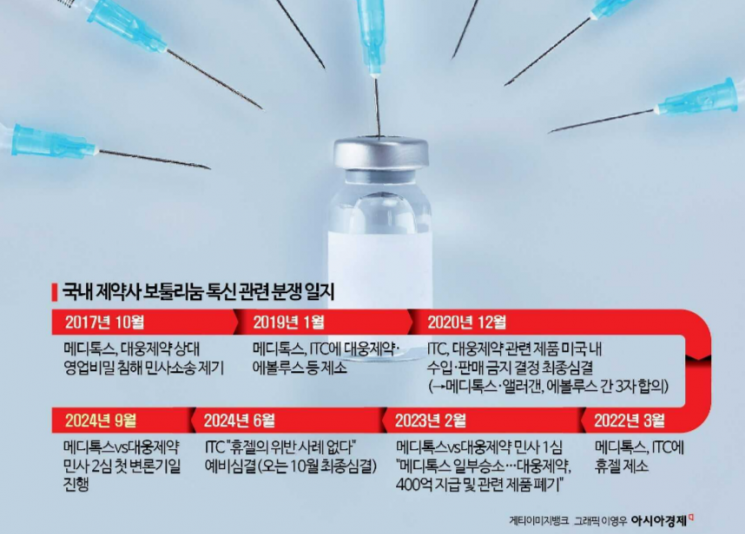
The second trial of the civil lawsuit filed by Medytox, claiming that Daewoong Pharmaceutical "stole the botulinum toxin strain," will officially begin next month. Botulinum toxin, commonly known as 'Botox,' causes a slight paralysis effect on muscles and is mainly used for wrinkle improvement procedures. The international botulinum toxin market size exceeded 9 trillion KRW as of 2022. In the previous trial, Daewoong Pharmaceutical was ordered to pay Medytox 40 billion KRW in damages and to dispose of finished products, among other rulings.
According to the legal community on the 13th, the Seoul High Court Civil Division 5-3 set the first hearing date for the appeal trial of the approximately 50 billion KRW trade secret infringement injunction lawsuit filed by Medytox against Daewoong Pharmaceutical for next month 26th. In May, the court held two preparatory hearings to coordinate the trial schedule. Preparatory hearings are procedures where representatives organize key issues in advance and plan necessary evidence and witness examinations to ensure smooth progress of the trial.
Before the appeal trial officially opens, both parties reorganized their legal teams and completed preparations for full-scale engagement. Daewoong Pharmaceutical appointed lawyers from Barun and Yulchon law firms as their representatives, while Medytox selected lawyers from HwaWoo, Gowon, and Sol law firms. The law firms Kwangjang, which represented Medytox in the first trial, and Sejong, which represented Daewoong Pharmaceutical, are not listed among the appeal trial representatives.
Previously, in 2017, Medytox filed this civil lawsuit on the grounds that Daewoong Pharmaceutical's botulinum toxin product 'Nabota' had stolen Medytox's 'Medytoxine.' In 2019, Medytox partnered with Allergan and filed a complaint against Daewoong Pharmaceutical and Evolus with the U.S. International Trade Commission (ITC). Evolus is Daewoong Pharmaceutical's partner company selling Nabota in the U.S., and at that time, Nabota had received approval from the U.S. Food and Drug Administration (FDA). The ITC banned the import of Nabota into the U.S. for 21 months at the end of 2020, but in February of the following year, Medytox and Allergan agreed to receive $35 million (approximately 56 billion KRW) from Evolus, concluding the ITC lawsuit.
Medytox also celebrated the first trial ruling in the domestic civil lawsuit issued last February. The first trial court stated, "It is difficult to deny the identity of the strains between Medytox and Daewoong Pharmaceutical," and ordered Daewoong Pharmaceutical to pay Medytox 40 billion KRW. Additionally, Daewoong Pharmaceutical was required to deliver the botulinum strains to Medytox and dispose of finished products. The use of related manufacturing technology was also prohibited.
Furthermore, the court pointed out, "Phylogenetic analysis results show that the strains of both parties are closely related," and "There is circumstantial evidence recognizing that Daewoong Pharmaceutical's strain originated from Medytox's strain. Daewoong Pharmaceutical shortened the development period by three months by acquiring the sequence arrangement information of Medytox's individual process steps."
The appeal trial is expected to feature intense disputes over whether the origin relationship between the two strains will be recognized. Immediately after the first trial ruling, Medytox welcomed it as "a clear judgment based on scientific evidence such as genome sequencing analysis that no one can refute," but Daewoong Pharmaceutical raised objections, stating, "Although it was acknowledged that the origin relationship cannot be judged solely by genetic analysis, the ruling was based on inference." However, as both parties applied for 'restricted access' due to concerns over confidential information leakage, the approximately 200-page first trial ruling has not yet been disclosed externally.
Subsequently, Daewoong Pharmaceutical filed a request for suspension of execution along with the appeal. Suspension of execution is a decision to temporarily halt the execution or effect of a disposition when it is recognized that irreparable damage may occur due to the specific disposition. Daewoong Pharmaceutical's suspension request was initially accepted by the court. Besides the civil lawsuit, Daewoong Pharmaceutical is also involved in three criminal cases arising from complaints and accusations by Medytox. Although investigative authorities initially dismissed the cases after a series of investigations, it is reported that the investigation procedures have resumed due to Medytox's objections and the results of the first civil trial.
Meanwhile, Medytox continues to engage in disputes related to botulinum toxin with the domestic company Hugel. In 2022, Medytox filed a complaint with the ITC against Hugel on the grounds that "Hugel also stole Medytoxine." The ITC concluded a preliminary ruling in June stating, "There is no violation regarding the import of botulinum toxin pharmaceuticals by Hugel into the U.S." Medytox immediately protested, and the final ruling in this case is expected to be issued in October.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.