Biosimilar Sales Grow 104%
US New Drug Zimpentrado Sales Reach 2.2 Billion
Operating Profit Declines Due to Merger Impact
Celltrion announced on the 7th that its consolidated earnings for the second quarter of this year amounted to KRW 874.741 billion in sales and KRW 72.488 billion in operating profit. Sales grew by 66.9% compared to the same period last year, surpassing KRW 800 billion in quarterly sales for the first time since the company's founding. However, operating profit decreased by 60.4% compared to the same period last year.
 Seo Jung-jin, Chairman of Celltrion Group (left), is conducting a Q&A session at the JP Morgan Healthcare Conference (JPMHC) held last January in San Francisco, California, USA.
Seo Jung-jin, Chairman of Celltrion Group (left), is conducting a Q&A session at the JP Morgan Healthcare Conference (JPMHC) held last January in San Francisco, California, USA. [Photo by Celltrion]
Celltrion evaluated that "the strong performance of existing biosimilar products and steady sales growth of follow-up products led to the increase in earnings." The core biosimilar business recorded sales of KRW 774 billion, a 103.6% increase year-on-year, driving the growth trend. Regarding operating profit, the company analyzed that it decreased due to "temporary cost ratio increases from inventory aggregation and amortization of intangible assets, as expected."
The company expects that sales and operating profit improvements will become more evident in the second half of the year. Following the merger with Celltrion Healthcare, the large intangible asset rights that had previously impacted profits were fully amortized as of this second quarter. Along with increasing sales and rapid depletion of existing inventory, the company explained that the cost of sales ratio will improve faster, leading to a full-scale rise in operating profit.
Through this, the company maintained its annual sales target of KRW 3.5 trillion for this year. The securities industry also forecasts that Celltrion will achieve sales of approximately KRW 3.5179 trillion this year.
Currently, Celltrion’s core biosimilar business is rapidly targeting the market by establishing a direct sales system in major global markets such as Europe and the United States, achieving stable market share growth. For example, Remsima, a biosimilar for autoimmune disease treatment, recorded a 59% market share in Europe in the first quarter of this year according to IQVIA, a pharmaceutical market research firm. When combined with Remsima SC, a subcutaneous injection formulation that improves dosing convenience, the combined market share in the five major European countries (EU5) stood solidly at 75%.
Celltrion also stated that other autoimmune disease treatment drug Upflima and oncology drugs Truxima, Herzuma, and Vegzelma continue to show growth, adding that "the direct sales systems in major global countries including Europe are stabilizing, and as successful bids increase in key countries, market share growth is expected to continue."
Celltrion is accelerating its efforts to penetrate the U.S. market with its next-generation growth engine, the autoimmune disease treatment new drug Jimptetra (the U.S. product name for Remsima SC). It recorded sales of KRW 2.2 billion in the U.S. in the second quarter alone. Recently, it succeeded in signing contracts with all major PBMs (Pharmacy Benefit Managers), which are the gatekeepers to the U.S. prescription market. Including contracts with other PBMs, Jimptetra currently covers about 75% of the U.S. insurance market. The company expects that coverage will further expand nationwide by the end of the year as it continues to pursue listings not only with major PBMs but also with numerous regional insurers.
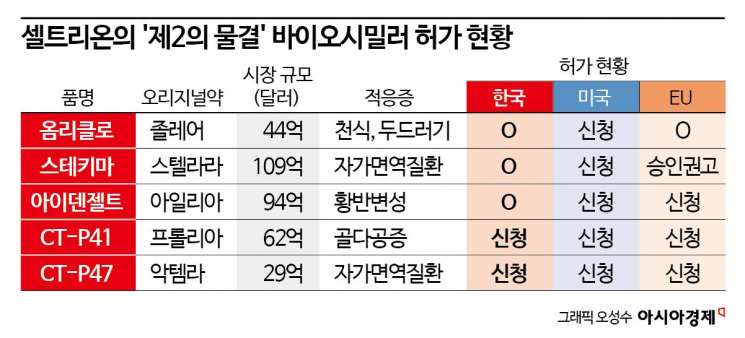
The approval process for follow-up pipelines after Jimptetra and the factory expansion work are also progressing smoothly. Celltrion previously announced the 'Second Wave' plan to complete a portfolio of 11 biosimilar products by 2025. Omriclo (Zolair biosimilar), Idenzel (Eylea biosimilar), and Stekima (Stelara biosimilar) have already begun receiving approvals in major countries, and CT-P41 (Prolia biosimilar) and CT-P47 (Actemra biosimilar) are currently undergoing approval procedures.
In terms of production capacity, the company explained that if the third plant, with an annual production capacity of 60,000 liters, which recently started its final verification process, begins commercial production within this year, it will enable 'small-volume multi-product production,' allowing flexible manufacturing of various items to meet the demand of follow-up pipelines.
A Celltrion official said, “With four products including the Remsima line, Upflima, and Vegzelma achieving record quarterly sales, and the next-generation engine Jimptetra generating actual sales in the U.S., we achieved the highest quarterly sales ever thanks to several positive factors. As temporary amortization factors related to the merger have been resolved and growth centered on follow-up products continues, a foundation for growth has been established. We will make company-wide efforts to realize a quantum jump this year.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














