A dementia treatment drug that targets the cause of early Alzheimer's disease has been approved domestically. Dementia requires various tests to be conducted alongside diagnosis and treatment, and with the growing need for side effect monitoring and early diagnosis, both domestic and international diagnostic industries are actively developing diagnostic technologies.
 Eisai and Biogen's Alzheimer's dementia treatment 'Leqembi (generic name: lecanemab)' [Image source=AP Yonhap News]
Eisai and Biogen's Alzheimer's dementia treatment 'Leqembi (generic name: lecanemab)' [Image source=AP Yonhap News]
The Ministry of Food and Drug Safety recently approved the dementia treatment drug Leqembi (lecanemab), jointly developed by Japan's Eisai and the U.S. company Biogen. Clinical trials demonstrated its efficacy in slowing cognitive decline in dementia patients by 27%. Korea Eisai, responsible for domestic sales, aims to supply the drug within the country by the end of this year. Since it is an expensive drug, sales growth will be difficult without health insurance coverage. Considering the indication is limited to mild cognitive impairment to mild dementia, the pharmaceutical industry expects that proving 'cost-effectiveness' during the reimbursement process will not be easy. In Japan, the annual health insurance price for the drug is set at 2.98 million yen (approximately 26 million KRW).
With the rapid increase in dementia patients due to aging populations, the domestic and international diagnostic industries expect the diagnostic market to grow alongside the approval of Leqembi, the first dementia causal treatment drug. The global number of dementia patients is projected to exceed 150 million by 2050, and market research firm Market Research Future forecasts that the Alzheimer's dementia diagnostic market size will grow at an average annual rate of 8.9%, increasing from $4.5 billion last year to $8.8 billion (approximately 12 trillion KRW) by 2032.
Dementia requires various diagnostic tests during confirmation and treatment. Alzheimer's dementia develops as proteins such as amyloid beta and tau clump in the brain, damaging brain cells. Levels of these proteins are assessed through cerebrospinal fluid (CSF) tests, positron emission tomography (PET), and magnetic resonance imaging (MRI) scans to determine the presence of dementia and treatment prognosis. Each method varies in cost, patient convenience, and equipment burden on medical institutions, so they are usually used in combination. Leqembi is prescribed to patients confirmed to have amyloid accumulation in the brain through PET scans.
Among these, MRI is expected to see market growth not only due to Leqembi but also as other treatment drugs currently under development become commercially available, to monitor side effects. Drugs like Leqembi remove amyloid attached to blood vessels, which can cause amyloid-related imaging abnormalities (ARIA) such as vascular bleeding and brain edema during the process. Regular brain MRI scans are required to check for abnormalities, and the Ministry of Food and Drug Safety mandates MRI checks before treatment initiation and before the 5th, 7th, and 14th doses. If ARIA appears, administration is paused, and subsequent MRI scans determine whether treatment can resume.
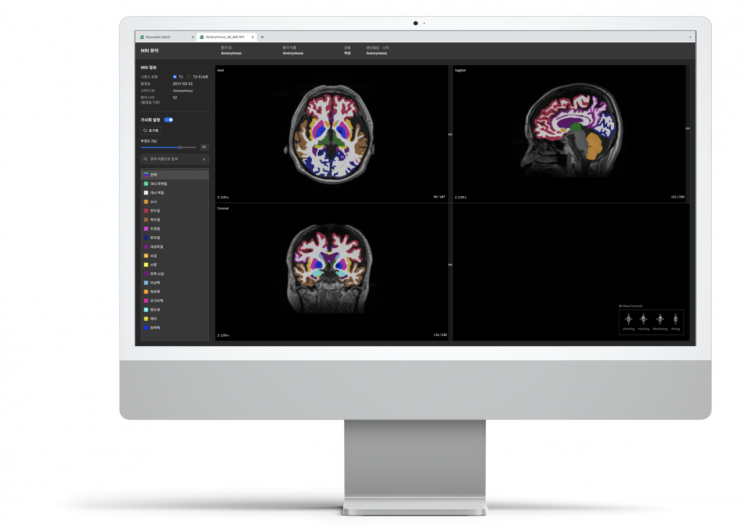
Domestic medical AI company Neurofit is developing AquaAD, a solution that supports the entire dementia diagnosis and treatment process. Using AI, it reduces the time for PET image analysis from 8 hours to 15 minutes and allows easy identification of brain region atrophy through MRI. Bin Jung-gil, CEO of Neurofit, said, "We plan to showcase this at next month's Alzheimer's Association International Conference (AAIC) and supply it mainly to major university hospitals expected to begin early Leqembi prescriptions," adding, "Our goal is a solution capable of handling everything from treatment prescription to prognosis monitoring across various imaging modalities such as PET and MRI." Vuno will also introduce its MRI-based degenerative brain disease diagnostic solution, Vuno Med-DeepBrain, to the U.S. market within this year. It quantifies the degree of atrophy in each brain region based on MRI images to assess patient status. Vuno Med-DeepBrain AD, specialized for Alzheimer's dementia, is also under development.
Since the prescription target for dementia drugs like Leqembi is early-stage dementia, early diagnosis is also gaining attention. In severe dementia stages where amyloid has excessively accumulated and brain cells are already destroyed, drug efficacy is difficult to expect, so confirming onset at the early symptom stage is necessary to achieve proper effects. The main method involves analyzing amyloid or tau concentrations in blood to indirectly assess the degree of protein aggregation in the brain. U.S. Roche Diagnostics is developing tau protein measurement technology in collaboration with Eli Lilly, which is developing the dementia drug donanemab. Domestically, companies such as PeopleBio, Quantamatrix, and Imocog are developing or marketing blood diagnostic technologies for dementia.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














