UNIST and Saudi KAUST Joint Research Team Develops Unified Catalyst Water Electrolysis Electrode
High Stability and Large-Scale Potential... Selected as Cover Paper in Journal of the American Chemical Society (JACS)
A new technology has been developed that overcomes the limitations of conventional catalytic electrodes, enabling the mass production of green hydrogen at a lower cost.
 [Research Team] Top row left: Professor Chan Ki Chae, top row right: Professor Jasper Yabuz, bottom row left: Professor Jong Beom Baek, from the third in the bottom row: first author Ga Hyun Lee, researcher Seok Jin Kim.
[Research Team] Top row left: Professor Chan Ki Chae, top row right: Professor Jasper Yabuz, bottom row left: Professor Jong Beom Baek, from the third in the bottom row: first author Ga Hyun Lee, researcher Seok Jin Kim.
A research team led by Professor Chae Hanki from the Department of Materials Science and Engineering and Professor Baek Jongbeom from the Department of Energy and Chemical Engineering at UNIST (President Lee Yonghoon), in collaboration with Professor Cafer T. Tavuz’s team at King Abdullah University of Science and Technology (KAUST), has developed a carbon fiber electrode with a high-performance catalyst attached.
Instead of using powder-type catalysts that easily detach, the team utilized a carbon fiber-type catalyst, allowing for stable operation over large areas. This enables the electrode to be used 100 times longer than conventional ones. By replacing expensive platinum with ruthenium, they maintained the same performance while significantly lowering the manufacturing cost.
Traditionally, powder-type catalysts such as carbon powder are sprayed and fixed onto the electrode during the manufacturing of electrochemical electrodes. However, it is difficult to apply the powder uniformly, leading to clumping or detachment, resulting in low stability and durability.
In contrast, electrochemical electrodes based on carbon fiber are attracting attention due to the high thermal and electrical conductivity of carbon fiber and the ease of scaling up to large areas.
The research team went a step further by immobilizing ruthenium on the surface during the production stage of the polymer precursor fiber, thereby enhancing the catalyst’s stability. The polymer precursor involved in the chemical reaction is polyacrylonitrile (PAN), which helps stably express the catalyst’s properties. Ruthenium was selectively attached to the surface as the chemical catalyst instead of platinum.
The Ru-SFC electrode, developed by selectively immobilizing ruthenium on the surface of the carbon fiber electrochemical fabric, exhibited a low overpotential of 11.9 mV at a current density of 10 mA cm?2. The lower the minimum voltage (overpotential) required for the hydrogen evolution reaction, the more energy-efficient the catalyst is.
Furthermore, while the overpotential of commercial platinum powder catalysts increased sixfold after 10,000 operations, the developed electrode showed only a 6.5% increase in overpotential. This means it can be used stably for 100 times longer.
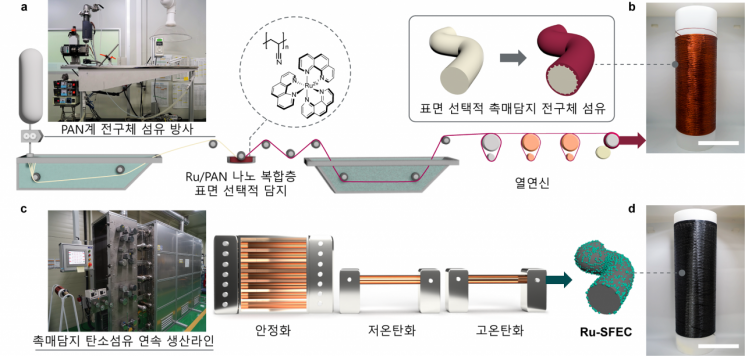 Schematic diagram of the fabrication of surface-selective catalyst-supported carbon fiber electrode.
Schematic diagram of the fabrication of surface-selective catalyst-supported carbon fiber electrode.
Professor Chae Hanki stated, "We utilized the inherent excellent mechanical and electrical properties of carbon fiber," adding, "This paves the way for carbon fiber to be used as a versatile material for electrochemical reactions in the future."
First author Lee Kahyun commented, "Through this research, we maximized stability and activity by controlling the separation of catalyst metals and the micro carbon structure," and added, "Since continuous catalyst fibers can be manufactured, it is immediately applicable in industry."
The electrode developed by the research team can be produced with less energy and generates less waste. The team validated the technology by expanding it to a continuous manufacturing process used in the actual carbon fiber industry.
The semi-pilot line continuous production method for catalyst-loaded carbon fiber secured in this study corresponds to Technology Readiness Level (TRL) 6. This represents a level of technology that can be implemented in real-world environments.
KAUST Research Professor Kim Seokjin, co-first author, explained, "Since this research is shaped into a flexible fiber form, it can be directly applied not only as an electrochemical catalyst but also as a thermochemical or photocatalyst."
The results of this study were officially published on May 15 in the Journal of the American Chemical Society (JACS), a globally renowned journal in the field of chemistry, and were selected as a supplementary cover image. Related patents and PCT applications were also completed on February 7, 2024 (Registration No./Date: 1026361690000 (2024.02.07)).
This research was conducted with support from the Ministry of Science and ICT and the Ministry of Education, and organized by the National Research Foundation of Korea through the Mid-career Researcher Support Program and the Leader Researcher Support Program.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














