Pemetrexed and Platinum-based Combination Therapy 'Ploura2'
mPFS 29.4 Months... Longest Among Related Therapies
Yuhan's 'Reclaza' Continues Dominance Battle Domestically and Abroad
Tagrisso, a lung cancer treatment, is striving to maintain its leadership in the lung cancer treatment market by gaining approval for the longest treatment duration combination therapy for the relevant genetic mutation, following its entry into the first-line treatment reimbursement market earlier this year.
 AstraZeneca's non-small cell lung cancer treatment 'Tagrisso (active ingredient Osimertinib)'
AstraZeneca's non-small cell lung cancer treatment 'Tagrisso (active ingredient Osimertinib)' [Photo by Korea AstraZeneca]
On the 16th, AstraZeneca Korea announced that the combination therapy of Tagrisso with pemetrexed and platinum-based (cisplatin, carboplatin, etc.) chemotherapy has been approved as a first-line treatment for lung cancer. Specifically, it is indicated for locally advanced or metastatic EGFR-mutated non-squamous non-small cell lung cancer with epidermal growth factor receptor (EGFR) exon 19 deletion or exon 21 (L858R) substitution mutations. The company emphasized that "Tagrisso is the first treatment to hold indications for both monotherapy and combination therapy in this regimen."
The approved regimen was first presented under the clinical trial name 'FLAURA2' at the International Association for the Study of Lung Cancer (IASLC) 2023 World Lung Cancer Congress (WLCC) in September last year and subsequently received approval from the U.S. Food and Drug Administration (FDA) in February.
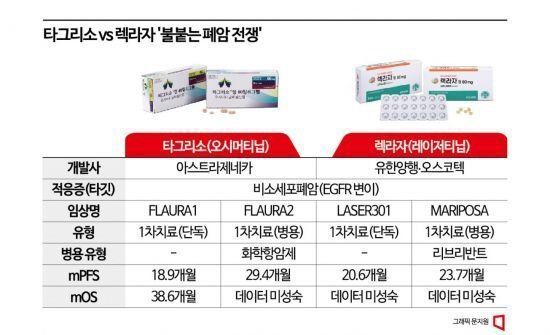
In the FLAURA2 trial, this regimen reduced the risk of disease progression or death (hazard ratio) by 38% compared to Tagrisso monotherapy. The median progression-free survival (PFS), a key measure of anticancer efficacy, was 25.5 months, extending 8.8 months longer than the 16.7 months observed with Tagrisso monotherapy. Independent central blinded review (BICR) reported median PFS of 29.4 months and 19.9 months, respectively.
PFS refers to the duration during which the cancer does not progress or recur. Along with overall survival (OS), which indicates the time from treatment initiation until death, it serves as a primary evaluation metric. Median values, representing the point at which more than half of the patients have reached the event, are typically used rather than averages for PFS and OS.
Professor Sang-Wi Kim, the principal investigator of the FLAURA2 trial in Korea and an oncologist at Seoul Asan Medical Center, stated, “Treating lung cancer patients with brain metastases or those harboring the L858R substitution mutation is very challenging and has poor prognosis, representing an unmet medical need. The clinical utility of the Tagrisso-chemotherapy combination therapy has been confirmed, allowing patients to choose one of two effective options based on Tagrisso. This domestic approval is therefore highly significant.”
Currently, Tagrisso is engaged in fierce competition for dominance with Lekraza, a lung cancer treatment developed by Yuhan Corporation and Oscotec and licensed to Johnson & Johnson (J&J). J&J reported a median PFS of 23.7 months in the 'Mariposa' clinical trial, which tested a combination therapy of Lekraza with their targeted antibody treatment Rybrevant rather than monotherapy. Although this extended the treatment duration compared to Tagrisso monotherapy, it did not surpass the FLAURA2 results. In Korea, competition had been limited to monotherapies since both Tagrisso and Lekraza entered the first-line treatment reimbursement market simultaneously in January, but with this approval, Tagrisso has gained an ally in combination therapy.
Mi-Sun Yang, Executive Director of Oncology Business at AstraZeneca Korea, said, “We are pleased that following Tagrisso’s first-line treatment health insurance reimbursement earlier this year, the Tagrisso-chemotherapy combination therapy has also been approved. This allows us to anticipate the efficacy of the combination therapy and reaffirms Tagrisso’s value as a global standard treatment.” She added, "As Tagrisso’s value as a global standard treatment for EGFR-mutated non-small cell lung cancer is reaffirmed, we will continue to strive to ensure that more patients with EGFR-mutated non-small cell lung cancer can benefit from Tagrisso treatment.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














