Global big pharma companies and domestic pharmaceutical firms are consecutively entering the development of glucagon-like peptide-1 (GLP-1) class obesity treatments.
Swiss global pharmaceutical company Roche recently signed a memorandum of understanding to acquire the US biotech company Camurus Therapeutics for $2.7 billion (approximately 3.545 trillion KRW). Roche is reportedly set to pay Camurus Therapeutics up to $400 million (about 525 billion KRW) in milestone payments.
Camurus Therapeutics is developing a GLP-1 receptor agonist drug candidate currently in Phase 2 clinical trials. The drug under development is being formulated in two types: oral (pill) and injectable. Separately, clinical trials are also underway for a candidate drug aimed at treating overweight diabetic patients.
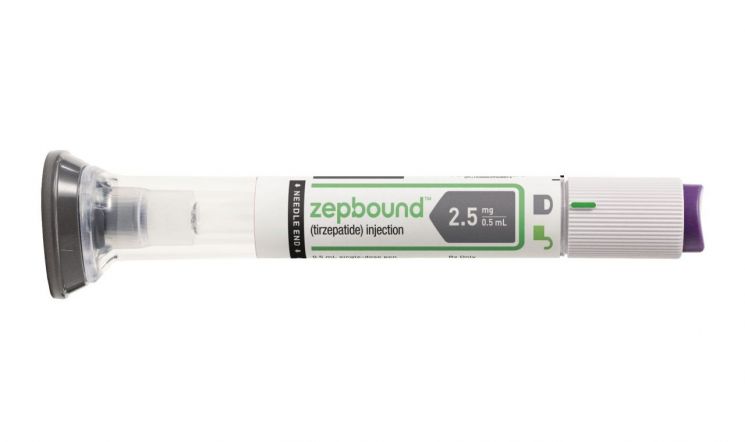
Eli Lilly’s GLP-1 class obesity treatment, Zepbound (active ingredient: Tirzepatide), began distribution in the US on the 5th of this month. Zepbound received approval from the US Food and Drug Administration (FDA) and the UK Medicines and Healthcare products Regulatory Agency (MHRA) on the 8th of last month. In clinical trials, after 72 weeks of administration, the treatment group showed a 24.5% weight reduction compared to the placebo group. Zepbound is the first drug to demonstrate over 20% weight loss in clinical trials. Zepbound contains the same amount of the active ingredient as Mounjaro, which Eli Lilly previously launched. Mounjaro was approved and released as a diabetes treatment in May last year but has also been prescribed off-label for obesity treatment due to its effectiveness.
Demand for already released products is also increasing. Danish pharmaceutical company Novo Nordisk’s obesity treatment, Wegovy (active ingredient: Semaglutide), is experiencing a global supply shortage. Wegovy was approved as an obesity treatment by the Korean Ministry of Food and Drug Safety in May but has not yet set a release date. In the Asian market, Japan is the only country where Wegovy’s launch is confirmed for February next year.
Currently developed treatments utilize GLP-1 analogs. These analogs extend the half-life of GLP-1, which suppresses appetite while slowing gastrointestinal motility, allowing food to remain longer in the intestines. The prolonged retention time increases the patient’s feeling of fullness. GLP-1 is also known to affect brain regions that regulate appetite and satiety. The global obesity treatment market, valued at approximately 4.3 trillion KRW last year, is expected to grow more than twice this year to 10.12 trillion KRW. Global investment bank Morgan Stanley forecasts the market size to reach $54 billion (about 71 trillion KRW) by 2030.
Among domestic companies targeting the GLP-1 obesity treatment market, Hanmi Pharmaceutical is leading. In October, Hanmi Pharmaceutical received approval from the Korean Ministry of Food and Drug Safety for the Phase 3 clinical trial plan of its GLP-1 obesity treatment, Epeglenatide (development code HM11260C). Epeglenatide requires only once-weekly administration. Hanmi Pharmaceutical aims to rapidly commercialize Epeglenatide as a “GLP-1 obesity treatment optimized for Koreans,” targeting domestic commercialization within three years.
Daewon Pharmaceutical and Daewoong Pharmaceutical are attempting to enter the obesity treatment market through formulation changes. Both companies are developing patch-type obesity treatments containing semaglutide, the main ingredient of Wegovy. Daewon Pharmaceutical submitted an Investigational New Drug (IND) application for the Phase 1 clinical trial of its patch-type obesity treatment ‘DW-1022,’ developed in collaboration with Korean biotech company Lapas, in August. Daewoong Pharmaceutical also announced plans to start Phase 1 clinical trials of its microneedle patch obesity treatment early next year and aims for commercialization by 2028.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














