Different Prices Compared to Original 'Humira'
Samsung Bioepis 'Hadlima' at 85% Discount
Celltrion 'Yuflyma' Only 5% Price Reduction
US Market Variables 'PBM' and 'Rebate' Impact
High Pricing Aims to Increase Market Share
Low Pricing Targets Public Insurance Market
The door to the U.S. market for Humira (active ingredient adalimumab) biosimilars (biopharmaceuticals) has finally opened. With the U.S. market valued at 24.5 trillion won, various prices are being set depending on strategies, and a fierce strategic battle is underway due to various factors such as concentration and side effect improvements during the product selection process.
On the 4th, Samsung Bioepis and Celltrion, domestic developers of Humira biosimilars, recently opened the gateway to the U.S. market. Samsung Bioepis, in partnership with its U.S. partner Organon, launched under the brand name "Hadlima" on the 1st, and Celltrion, which developed the product, with Celltrion Healthcare handling overseas sales, released "Yuflyma" on the 2nd. Notably, the Celltrion Group's chairman Seo Jung-jin and other executives are reportedly engaging in direct sales activities in the U.S. market.
What is unusual is the significant price difference between the two drugs. Generally, biosimilars are known to improve patient accessibility by offering lower prices. Organon, the distributor of Hadlima, set the wholesale acquisition cost (WAC) at $1,038 (about 1.36 million won), an 85% reduction compared to Humira's monthly price of $6,922 (about 9.05 million won), aligning with this perception. On the other hand, Celltrion Healthcare priced Yuflyma's WAC at $6,576.5 (about 8.6 million won), only 5% lower than the original. This results in a monthly cost difference of about $5,500 even between biosimilars.
The same applies to other biosimilars. Coherus's "Yusimri" also adopted a low-price competition strategy by setting a WAC of $995 (about 1.3 million won), an 85.6% reduction compared to the original, similar to Samsung Bioepis. Meanwhile, Boehringer Ingelheim priced "Siltjezo" at 5-7% lower than the original, similar to Celltrion Healthcare's level. Some companies have introduced a mixed strategy. Alongside drugs with typical discount levels, they offer "unbranded" products sold only under the generic name or active ingredient name with significantly larger discounts instead of official brand names. Amgen, which first launched "Amjevita" in January, set a basic discount of 5% but applied a 55% discount to the unbranded version, and Sandoz, which developed "Hyrimoz," also released an unbranded product with an 81% discount in addition to the basic 5% discount.
Key Variables in the U.S. Market: 'PBM' and 'Rebates'
This situation stems from the fact that the structure of the U.S. pharmaceutical market is very different from that of Korea. There exists a special institution called Pharmacy Benefit Managers (PBMs), and rebates, which are illegal in Korea, are allowed in the U.S. Depending on how these two factors are viewed in terms of their impact on the market, prices are set either low, high, or follow a two-track strategy.
PBMs negotiate drug prices and rebate levels with pharmaceutical companies on behalf of insurers and manage formularies, which are lists of drugs that can actually be prescribed at pharmacies. Many public insurance programs also outsource these tasks to PBMs. For specialty drugs like Humira, it is virtually impossible to sell them at pharmacies unless they are listed on PBM formularies. Therefore, the number of formularies on which a product is listed and the tier level it occupies determine the product's success or failure. Currently, the U.S. Humira PBM market is an oligopoly with three major PBMs?CVS Caremark (33%), Express Scripts (24%), and OptumRx under UnitedHealth Group (22%)?holding about 80% market share. Success in getting listed on their formularies can significantly increase market share.
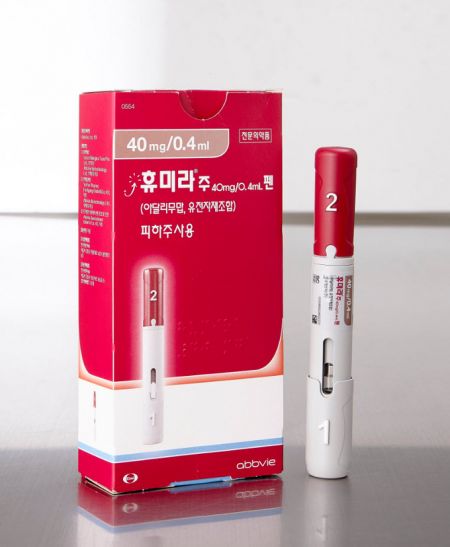
 Celltrion's Humira biosimilar 'Yuflyma'. The seller, Celltrion Healthcare, set the wholesale price of Yuflyma at $6,576.5, which is 5% lower than the original.
Celltrion's Humira biosimilar 'Yuflyma'. The seller, Celltrion Healthcare, set the wholesale price of Yuflyma at $6,576.5, which is 5% lower than the original. [Photo by Celltrion Healthcare].jpg
Those adopting a high-price strategy appear to prioritize listing on these major PBMs. Since rebates paid to PBMs are calculated as a percentage of the drug price, higher prices can provide PBMs with larger rebates. From the PBM perspective, if the active ingredient is the same, drugs with smaller discounts should sell more to maximize their profits. In fact, Amjevita, Hyrimoz, and Siltjezo, which OptumRx reportedly adopted in private insurance formularies excluding Hadlima and Yuflyma, all lowered their basic WAC by only about 5%.
Additionally, recent U.S. government measures to recoup excess profits when drug prices for public insurance-covered drugs rise above inflation have started to impose restrictions, which is also considered a factor for choosing higher prices. While price increases are limited, there are no special regulations on price reductions, so it seems that only small discounts were applied at launch to allow for flexible pricing policies in response to future market and policy changes.
Bioepis Adopts 'Ultra-Low Price' Strategy...Possibility of Pressuring PBMs
On the other hand, Organon and Coherus have adopted an ultra-low price strategy with about an 85% discount. Kim Min-jung, a researcher at DS Investment & Securities, said about these drugs, "Compared to big pharma companies with high rebate strategies, they will inevitably offer very minimal rebates," and "The low WAC price will inevitably lose some rebate competitiveness." However, she also noted, "They may exert policy pressure on PBMs," and "PBMs will need to explain why they do not cover products that are more than 85% cheaper," suggesting the possibility of a counterattack.
 Samsung Bioepis's Humira biosimilar 'Hadlima'. Its sales partner, Organon, launched Hadlima in the market at a wholesale price of $1,038, which is a remarkable 85% lower than the original.
Samsung Bioepis's Humira biosimilar 'Hadlima'. Its sales partner, Organon, launched Hadlima in the market at a wholesale price of $1,038, which is a remarkable 85% lower than the original. [Photo by Samsung Bioepis]
Kim also cited the biosimilar "Semglee" of basal insulin "Lantus" (active ingredient insulin glargine) as an example, noting that a low-price strategy can also be sufficiently competitive. Semglee, developed by Viatris, was launched in 2021 at a price not significantly different from the original. Subsequently, it succeeded in being included in the largest formulary of Express Scripts, excluding the original, but was not listed on other major PBM formularies. Last year, Semglee held only a 29% market share in the commercial market based on total prescriptions (TRx).
However, the notable point is the unbranded version of Semglee, insulin glargine. Using the active ingredient name as the product name, it was launched at a 65% discount at $148. Insulin glargine had a 19% market share in the commercial market last year and a 30% TRx share in Medicaid, a public insurance program for low-income groups, far surpassing Semglee's 3%. This indicates that a low-price strategy may be preferred by public insurance and regional medical institutions, and some PBMs tend to prefer lower prices over rebates. The Mark Cuban Cost Plus Drug Company (MCCPDC), which partnered with Coherus to sell Yusimri, is a public benefit corporation established to provide affordable generics and biosimilars to the public regardless of health insurance.
Quality Also a Variable...Must Secure 'High Concentration' and 'Interchangeability' for Competitiveness
Quality is also a key variable. Humira is available in a low concentration formulation of 50 mg/mL and a high concentration formulation (HCF) of 100 mg/mL, which reduces the drug dosage by half. As of last year, 85% of adalimumab sold in the U.S. was HCF, indicating a much higher preference for HCF. This is analyzed as the reason why Amjevita, which has only developed the low concentration product, has a market share of less than 1% even after about six months since launch. Meanwhile, Yuflyma was developed only as HCF, and Hadlima has both low concentration and HCF. Besides these, only Hyrimoz has both low concentration and HCF, while other companies are either still developing or have entered the market with only low concentration products.
Another quality variable is "interchangeability." Due to the nature of biopharmaceuticals, which are difficult to produce identically, biosimilars demonstrate "similarity" rather than equivalence. This is why cross-substitution is not allowed for biosimilars, unlike generics (synthetic drug copies). However, if interchangeability is recognized, pharmacists can substitute the biosimilar for Humira when dispensing to patients prescribed Humira.
Currently, only Siltjezo has been recognized as interchangeable with Humira. Other biosimilars like Hadlima and Yuflyma are undergoing development to secure interchangeability. Samsung Bioepis completed clinical trials in May and is analyzing related data, stating, "We will strive to complete the remaining procedures in a timely manner." Celltrion is also conducting interchangeability clinical trials in Estonia with 366 patients with plaque psoriasis. However, since interchangeable biosimilars enjoy exclusive status for one year after launch, even if interchangeability is recognized, actual substitution is expected to be possible only after July next year.
However, it is pointed out that the impact of interchangeability is not very significant in the U.S. market, which operates under the PBM structure. For patients insured through formularies that do not list Siltjezo, insurance coverage is not possible for Siltjezo even if interchangeability is recognized. If substitution with Siltjezo occurs at pharmacies, patients may end up bearing the full cost of the drug.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














