Issue 34, Carlos Medical 'Dineksu'
Issue 35, Imocog 'Cogtera'
The Ministry of Food and Drug Safety announced on the 21st that it has designated Carlos Medical's radiofrequency energy treatment device for hypertension treatment, 'DineX,' and Imocog's cognitive therapy software for mild cognitive impairment (MCI) treatment, 'Cogthera,' as the 34th and 35th innovative medical devices, respectively.
 High-frequency energy treatment device for hypertension 'DineX' by Carlos Medical [Photo provided by the Ministry of Food and Drug Safety]
High-frequency energy treatment device for hypertension 'DineX' by Carlos Medical [Photo provided by the Ministry of Food and Drug Safety]
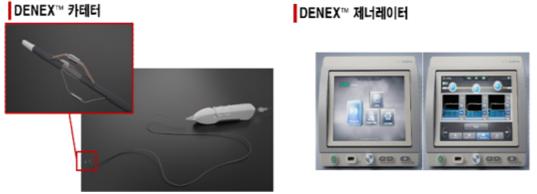
DineX, composed of a 'disposable pulse-controlled electrosurgical electrode' and a 'universal electrosurgical device,' is the first domestic product that treats patients with hypertension uncontrolled by drug therapy using radiofrequency energy. The electrode catheter is inserted into the renal artery to deliver radiofrequency energy to the target area, blocking the sympathetic nerves distributed on the outer wall of the blood vessels to treat hypertension. Since chronic diseases such as hypertension, heart failure, and renal failure are caused by abnormal sympathetic nervous system hyperactivity, the mechanism involves blocking the sympathetic nerve bundles passing around major organs with radiofrequency energy for treatment.
The Ministry of Food and Drug Safety explained that it designated the device as an innovative medical device as it is expected to be a new treatment method for patients with resistant hypertension who do not respond to drug therapy. Currently, confirmatory clinical trials are underway. Unlike general pharmaceuticals, medical devices undergo two stages of clinical trials for approval: exploratory clinical trials and confirmatory clinical trials. Exploratory clinical trials, the initial stage, are conducted over a relatively short period with a small number of clinical trial subjects, while confirmatory clinical trials, conducted at the product development stage, involve a statistically significant number of clinical trial subjects.
 Imocog's digital therapeutic device (DTx) for mild cognitive impairment treatment, 'Cogthera'
Imocog's digital therapeutic device (DTx) for mild cognitive impairment treatment, 'Cogthera' [Photo by Imocog]
Imocog's Cogthera is a digital therapeutic device (DTx) that provides patient-tailored training based on cognitive intervention therapy (CIT) to improve cognitive function in MCI patients experiencing memory decline. There are no similar domestic approval cases yet.
Cogthera periodically monitors the patient's cognitive function status and provides patient-customized cognitive training with difficulty levels based on metacognitive training, along with weighted training for deficient cognitive areas, thereby enhancing cognitive reserve and helping improve cognitive function. Cognitive reserve is a concept similar to a storage warehouse for cognition and improves the more the brain is used. Through this, cognitive decline can be delayed or cognitive function improved.
The Ministry of Food and Drug Safety stated that it designated Cogthera as an innovative medical device in recognition of its technological innovation, such as digitalizing cognitive intervention therapy previously conducted in hospitals to enable treatment at home.
Cogthera is currently conducting confirmatory clinical trials, having registered its first patient on the 2nd. The trials are planned to be conducted on 100 patients across six hospitals nationwide, with clinical completion targeted for next year.
An official from the Ministry of Food and Drug Safety said, "So far, a total of 35 products have been designated as innovative medical devices," adding, "The operation of this innovative medical device designation system is expected to contribute to realizing the government's national agenda of becoming a global hub for bio and digital health." He continued, "We will continue to make efforts to provide safe and new treatment technologies to the public quickly through close support such as priority reviews to support the rapid commercialization of innovative medical devices."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














