P-CAB Class GERD New Drug
Export Contract with Moroccan Pharma Company Cooperpharma
Approved in 3 Global Countries Within a Year of Launch
Daewoong Pharmaceutical's new drug for gastroesophageal reflux disease, 'Pexuclu,' is accelerating its overseas expansion by signing an export contract to Africa.
On the 19th, Daewoong Pharmaceutical announced that it had signed an export contract for the potassium-competitive acid blocker (P-CAB) new drug 'Pexuclu' (active ingredient: Pexuprazan) with Cooper Pharma, a local pharmaceutical company in Morocco. The contract is valued at $20.32 million (approximately 27 billion KRW), with plans to launch Pexuclu locally in 2025. Morocco, considered the largest pharmaceutical market in North Africa, has a gastroesophageal reflux disease treatment market worth 75.5 billion KRW, all of which currently consists of proton pump inhibitor (PPI) drugs. Pexuclu will be the first P-CAB formulation introduced in Morocco.
Founded in 1933, Cooper Pharma has rapidly expanded its business in the pharmaceutical industry and maintains the number one market share in Morocco's gastroesophageal reflux disease market. Daewoong Pharmaceutical plans to leverage Cooper Pharma's local market dominance and hospital and clinic networks to quickly replace PPI drugs with the P-CAB class drug Pexuclu. Furthermore, the strategy is to change the treatment paradigm for gastroesophageal reflux disease across the African continent, starting with Morocco.
P-CAB drugs are rapidly replacing existing proton pump inhibitors (PPIs) both domestically and internationally by emphasizing their ease of administration compared to conventional drugs. PPIs, which have been primarily prescribed for gastroesophageal reflux disease, must be taken 30 minutes before meals and still allow acid secretion during sleep, causing nighttime heartburn symptoms. In contrast, P-CAB drugs can be taken regardless of meal times and have relatively faster efficacy. Suppression of acid secretion also improves nighttime heartburn.
Pexuclu, which has been on the domestic market for less than a year, has already received product approvals in three foreign countries, accelerating its global market penetration. Daewoong Pharmaceutical launched Pexuclu in the domestic market in July last year. Just four months after its launch, in November last year, it received its first overseas product approval from the Philippine Food and Drug Administration. Subsequently, in February and March of this year, it obtained approvals from the authorities in Ecuador and Chile, respectively. Currently, approval applications have been submitted and related procedures are underway in eight countries, including Brazil, Mexico, Peru, and Vietnam.
The company is also increasing the pace of entry into global big markets. On the 5th, Daewoong Pharmaceutical announced that it had agreed to terminate the exclusive license agreement with Neurogastrix, a U.S. pharmaceutical company holding clinical and commercialization rights for Pexuprazan in the U.S. and Canada. At the same time, it has entered negotiations with several multinational pharmaceutical companies capable of conducting clinical trials and development in large markets such as North America, Europe, and Japan. This is interpreted as a strategy to accelerate entry into major global markets, including North America, through contracts with new partners.
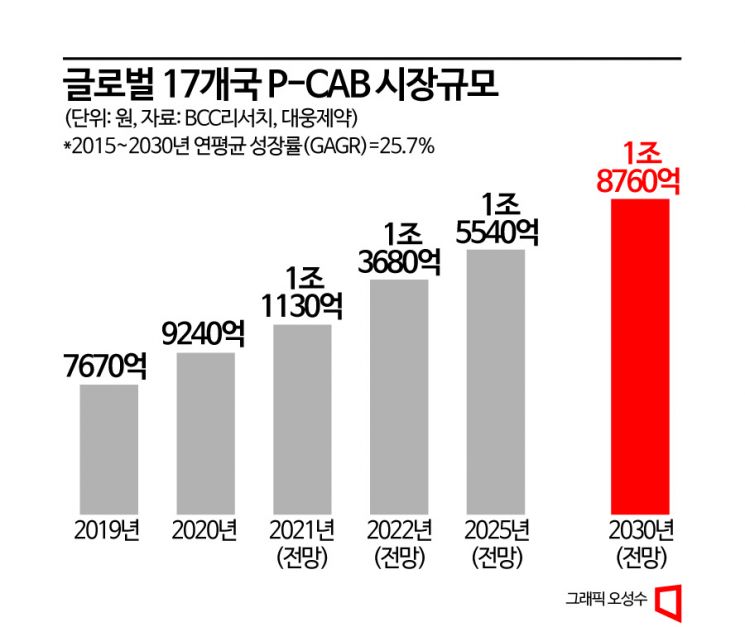
Daewoong Pharmaceutical's acceleration of Pexuclu's overseas expansion is analyzed to be due to the global market size for P-CAB drugs showing annual growth. According to BCC Research, a global research institute, the P-CAB market in 17 major countries is expected to grow from 61 billion KRW in 2015 to 1.876 trillion KRW by 2030. The compound annual growth rate during this period is projected to reach 25.7%.
Seungho Jeon, CEO of Daewoong Pharmaceutical, said, "It is encouraging that Pexuclu's reputation is rising in the global gastroesophageal reflux disease treatment market and that it has expanded to the African continent in less than a year since its domestic launch. Daewoong Pharmaceutical will continue to promote Pexuclu's strengths and nurture it into a global blockbuster."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.


![User Who Sold Erroneously Deposited Bitcoins to Repay Debt and Fund Entertainment... What Did the Supreme Court Decide in 2021? [Legal Issue Check]](https://cwcontent.asiae.co.kr/asiaresize/183/2026020910431234020_1770601391.png)













