Interview with Noh Yuhun, CEO of Imocog
Developing all solutions including selection, diagnosis, treatment, and management
Beyond digital to blood diagnostics
"Aiming for a bio-digital platform"
Digital therapeutics (DTx) developers typically look for other indication pipelines based on the completion of one DTx development. However, Imocog, which is developing the mild cognitive impairment (MCI, a pre-dementia stage) treatment DTx 'Cogthera,' has chosen a different path.
Cogthera received approval for its confirmatory clinical trial in South Korea last October, and the first patient was enrolled on the 2nd. The clinical trial is being conducted at six hospitals nationwide with about 100 participants, aiming to complete the trial by next year. Although the first pipeline is on track, Imocog is focusing not on finding other indications but on MCI and dementia, promoting the provision of a 'full-cycle dementia solution.'
On the 2nd, when the first patient enrollment for Cogthera's confirmatory clinical trial took place, No Yooheon, co-CEO of Imocog, told Asia Economy, “As Cogthera development progressed, we considered what the next development strategy should be,” adding, “We believed it was more appropriate to have a full-cycle dementia pipeline that could create synergy with Cogthera rather than pursuing other indications.”
Significant achievements have already been made. In the dementia screening and diagnosis stages, the technologies for 'Cogscreen' - 'Cognosis' - 'Cogcheck' have been completed, and the therapeutic DTx Cogthera has entered confirmatory clinical trials. Additionally, a cognitive training program for seniors called 'Cogcare' has also been introduced.
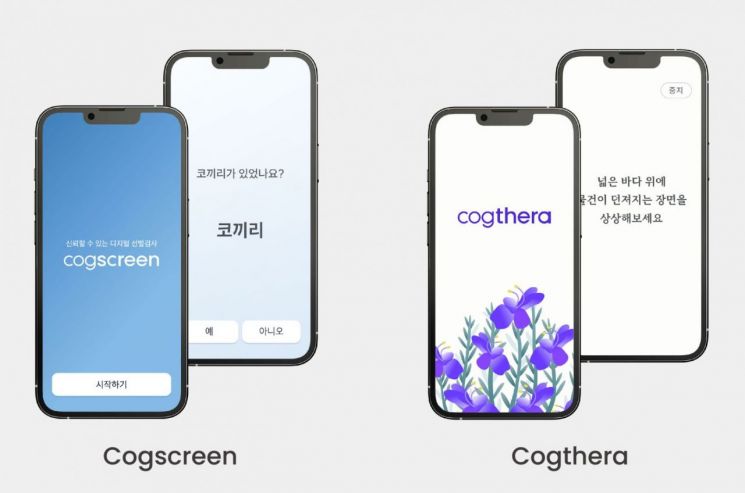 Imocog's dementia screening tool 'CogScreen' (left) and digital therapeutic device (DTx) 'CogThera'
Imocog's dementia screening tool 'CogScreen' (left) and digital therapeutic device (DTx) 'CogThera' [Photo by Imocog]
In Imocog's dementia treatment ecosystem, early screening is conducted within about 10 minutes through Cogscreen when MCI is suspected. If cognitive decline is suspected here, diagnosis proceeds through Cognosis. Previously, nurses or other personnel had to conduct verbal diagnostic tests for 40 to 90 minutes, but Cognosis allows testing via smartphone in 40 minutes, with immediate results. CEO No said, “It was difficult for local clinics or rural areas, where manpower is hard to manage, to provide dementia diagnosis,” adding, “With Cognosis, diagnosis can be conveniently received anywhere.”
If cognitive abnormalities are detected here as well, the process moves on to blood testing. When amyloid-beta (Aβ) protein, known as the main cause of Alzheimer's disease, accumulates in the brain, Aβ also dissolves into the blood. Recently, technology using this as a biomarker to diagnose dementia has been commercialized and is gaining attention. Imocog has developed and commercialized the blood diagnostic technology 'Cogcheck' and ultimately plans to use AI analysis of the entire blood profile, not just Aβ, as biomarkers. CEO No explained, “This is why we describe Imocog as a 'bio-digital healthcare platform company,'” adding, “We want to create services that integrate bio technologies such as blood diagnostics.”
Especially with dementia antibody treatments like Aduhelm and Leqembi nearing commercialization, the potential of Cognosis, which can diagnose dementia subtypes, is highly regarded. About 70% of dementia patients suffer from Alzheimer's dementia, so 'dementia' is usually associated with Alzheimer's dementia. Conversely, 30% suffer from other types such as vascular, Lewy body, or alcoholic dementia. For them, current antibody treatments that remove Aβ to suppress cognitive decline are unlikely to be effective. Therefore, using Cognosis to selectively identify Alzheimer's patients enables personalized treatment by administering antibody therapies only to them.
The 'selection and focus' approach aligns with Cogthera's development, which is concentrated on cognitive intervention therapy (CIT). The globally widely used FINGER program for MCI and dementia treatment is a multi-modality program covering five areas: diet, exercise, social interaction, and cardiovascular risk. However, Imocog's Cogthera focuses solely on cognitive training. CEO No said, “Considering the characteristics of DTx as software medical devices (SaMD), we thought focusing on cognitive therapy was appropriate,” adding, “Accessibility was also an important consideration.” He added, “Exercise requires screen sizes like TV or tablets, which impose additional economic burdens, and managing diet through DTx is not easy.” In this regard, since MCI patients are likely to have low digital literacy, Cogthera's app is designed to be as simple as possible, conducting most treatments verbally to enhance convenience.
Instead, other modalities are planned to be provided through Cogcare, a service linked with DTx. For exercise, memory improvement services through physical activity are offered via MediBallet's 'Uwadance,' in collaboration with the Professional Dancer Support Center, and cognitive improvement continues offline through multi-strategy memory training called 'Memory Care.'
Imocog is also actively exploring overseas expansion. It is the only dementia and MCI-related DTx developer that is a member of the global Digital Therapeutics Alliance (DTA). CEO No said, “Because dementia-related DTx developers are rare worldwide, interest in Imocog is growing,” adding, “We are considering expansion into Germany, the United States, and other countries.”
To this end, last year, Imocog established its German branch, 'Cogthera GmbH,' in Munich. Germany is recognized as a country operating the most innovative DTx policy through the Digital Health Application (DiGA) system. Regardless of proof of treatment effectiveness, manufacturers' proposed prices are listed for insurance reimbursement for one year, with prices negotiated based on performance afterward, making it an optimal region for DTx developers to seize opportunities. CEO No said, “Our goal is to enter DiGA by next year,” adding, “We aim to create the world's first cognitive improvement DTx.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.