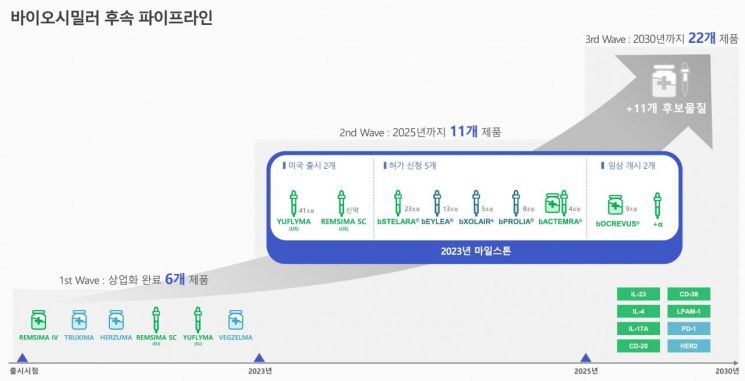
Pipeline Doubled Following Completion of 11 Developments
Recent Clinical Launch of 'Okrebus' Biosimilar
Major Blockbuster Biosimilars in Oncology and Autoimmune Diseases Expected
Celltrion has officially launched its 'Third Wave (3rd Wave)' strategy to establish itself as a global leader in biosimilars by building an additional pipeline of 11 blockbuster biosimilars (biopharmaceutical generics) by 2030.
The first step is the biosimilar of the multiple sclerosis treatment 'Ocrevus,' which has annual sales reaching 9 trillion KRW, called 'CT-P53.' Following the European Medicines Agency (EMA) last month, the company submitted a clinical phase 3 trial plan (IND) to the U.S. Food and Drug Administration (FDA) on the 15th (local time). The clinical trial will last 96 weeks and compare CT-P53 with Ocrevus in 512 patients with relapsing-remitting multiple sclerosis, an autoimmune disease.
Ocrevus is the top-selling treatment in the global multiple sclerosis market, generating sales of 6.036 billion Swiss francs (approximately 9 trillion KRW) as of last year. Its patent is expected to expire in the U.S. in 2029. Celltrion plans to complete development in time for the patent expiration and, after starting development in 2021, aims to enter phase 3 clinical trials soon with this IND approval. So far, only Poland's Polpharma has announced plans to develop a biosimilar, and since it is still in the early development stage, Celltrion expects to take the lead as the 'first mover.'
Currently, Celltrion has completed development of a total of 11 biosimilars. Starting with 'Remsima IV,' the world's first antibody biosimilar for autoimmune disease treatment, six biosimilars including 'Remsima SC,' 'Yuflyma,' and anticancer drugs 'Truxima,' 'Herzuma,' and 'Vegzelma' have already been commercialized. In addition, development of five biosimilars?'Xolair,' 'Stelara,' 'Eylea,' 'Prolia,' and 'Actemra'?has been completed, and approvals are being prepared or underway. The plan is to double the pipeline by adding a total of 11 candidate substances within the next eight years.
 Celltrion has developed the world's first intravenous antibody biosimilar 'Remsima IV'
Celltrion has developed the world's first intravenous antibody biosimilar 'Remsima IV' [Photo by Celltrion]
Specific targets have already been announced. In the oncology field, these include programmed cell death protein (PD)-1, human epidermal growth factor receptor (HER)2 and HER2 antibody-drug conjugates (ADC), and in the autoimmune disease field, interleukin (IL)-23, IL-4, IL-17A, CD-20, CD-38, and LPAM-1. Among these, the biosimilar CT-P53 of Ocrevus, an autoimmune disease treatment that binds to B-cell CD20 to suppress immune responses, was the first to be revealed. Since other targets are also blockbusters with high sales in the market, Celltrion plans to continue its first mover strategy by launching biosimilars quickly in line with patent expiration dates.
The largest market is for anti-PD-1 immune checkpoint inhibitors. In South Korea alone, Merck (MSD)'s 'Keytruda' (generic name pembrolizumab), which has been approved for 12 cancer types and 22 indications including melanoma, non-small cell lung cancer, triple-negative breast cancer, and cervical cancer, dominates the oncology market. It is expected to generate sales of $24 billion (approximately 32 trillion KRW) this year and is projected to become the world's top-selling drug. Other immune checkpoint inhibitors such as Bristol Myers-Squibb (BMS)'s 'Opdivo' are also expanding the market, with the total anti-PD-1 drug market expected to reach $58 billion (approximately 78 trillion KRW) by 2025. If Celltrion succeeds in developing biosimilars of these drugs, explosive sales growth is anticipated.
In addition, in the oncology field, besides Celltrion's biosimilar 'Herzuma' of 'Herceptin,' Roche's HER2-targeting anticancer drug 'Perjeta,' which recorded sales of 4.1 billion Swiss francs (approximately 6 trillion KRW) last year, and AstraZeneca-Daiichi Sankyo's HER2-targeting ADC anticancer drug 'Enhertu,' recently approved in South Korea for its innovative efficacy, are considered promising biosimilar development targets. ADCs have recently attracted significant attention in the industry as a next-generation modality (therapeutic approach), and Celltrion has also pursued ADC technology acquisition through collaborations with Exicure Therapeutics and Pinova Bio.
In the autoimmune disease field, the IL-4 inhibition market is the largest. Sanofi's 'Dupixent,' used for atopic dermatitis and asthma treatment, recorded sales of 8.3 billion euros (approximately 12 trillion KRW) last year. Dupixent is also expected to continue its growth trend, with sales projected to exceed 10 billion euros this year.
Celltrion plans to continue this biosimilar growth strategy while also establishing itself as a new drug development company. At a recent press conference, Seo Jung-jin, Chairman of the Celltrion Group, stated, "By 2030, we aim to increase the proportion of original new drug sales to 40%," adding, "We will be a leader in biosimilars and also compete shoulder to shoulder with multinational companies as a new drug developer." In addition to biosimilars, the company plans to initiate clinical trials for six bispecific antibody new drugs and ten anticancer new drugs within next year.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.


![User Who Sold Erroneously Deposited Bitcoins to Repay Debt and Fund Entertainment... What Did the Supreme Court Decide in 2021? [Legal Issue Check]](https://cwcontent.asiae.co.kr/asiaresize/183/2026020910431234020_1770601391.png)













